Application of a class of compounds in the preparation of anti-picorna virus drugs
A compound and drug technology, applied in the field of medicine, can solve problems such as poor antiviral effect, and achieve the effect of shortening the disease process, strengthening the protective effect, and protecting the nervous system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
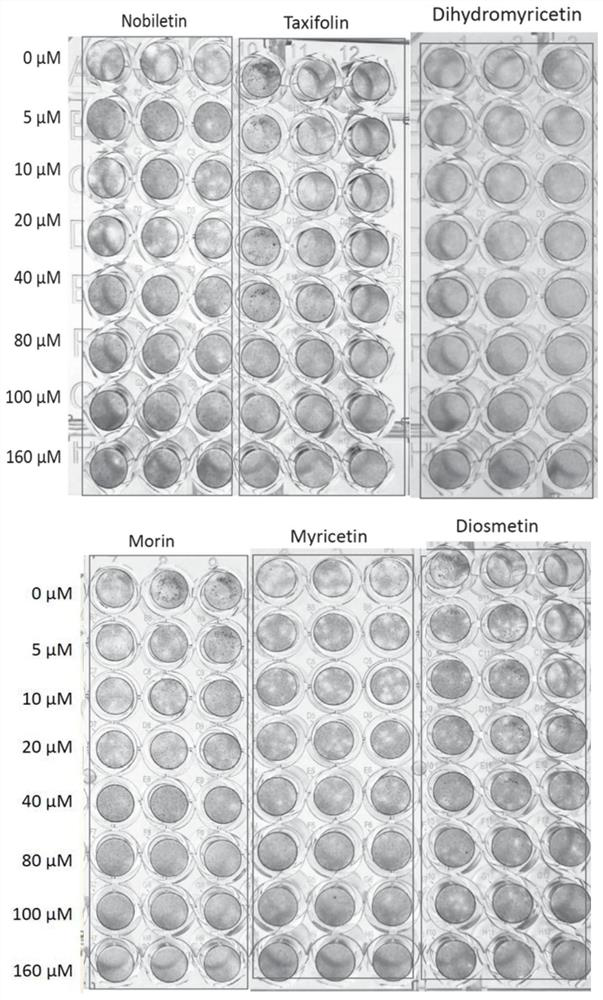
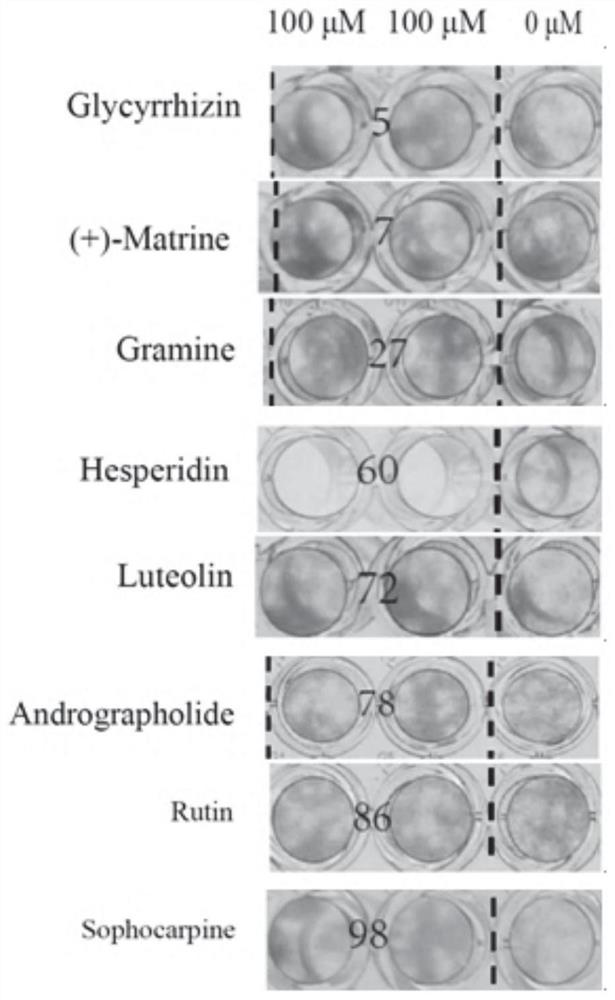
[0126] Embodiment 1 Drug Gradient Inhibition Experiment
[0127] RD (human malignant embryonic rhabdomyoma cells) cells were divided into 1×10 4 Cells / well were seeded in 96-well tissue culture plates (Corning, USA) and incubated at 37°C, 5% CO 2 Incubate in atmosphere for 3 days until approximately 100% confluency is reached. After forming a monolayer of RD cells at the bottom of the well, add 60 μL of diluted virus stock solution GZ203ACL21 with an appropriate titer to each well of the 96-well plate (2-8 plaques can be formed in the control well). Then place the plate at 37 °C, 5% CO 2 After incubation for 1 hour in the incubator, overlay medium was added directly to each well. Dilute 200 mM drug (dissolved in DMSO) with DMEM medium to 1 mM concentration. Then, 1.6 μL, 3.2 μL, 6.4 μL, 12.8 μL, 16 μL, and 25.6 μL of the compound were directly added to the covering medium of each well (3-6 duplicate wells). at 37°C, 5% CO 2 After culturing in the incubator for 2 days, pl...
Embodiment 2
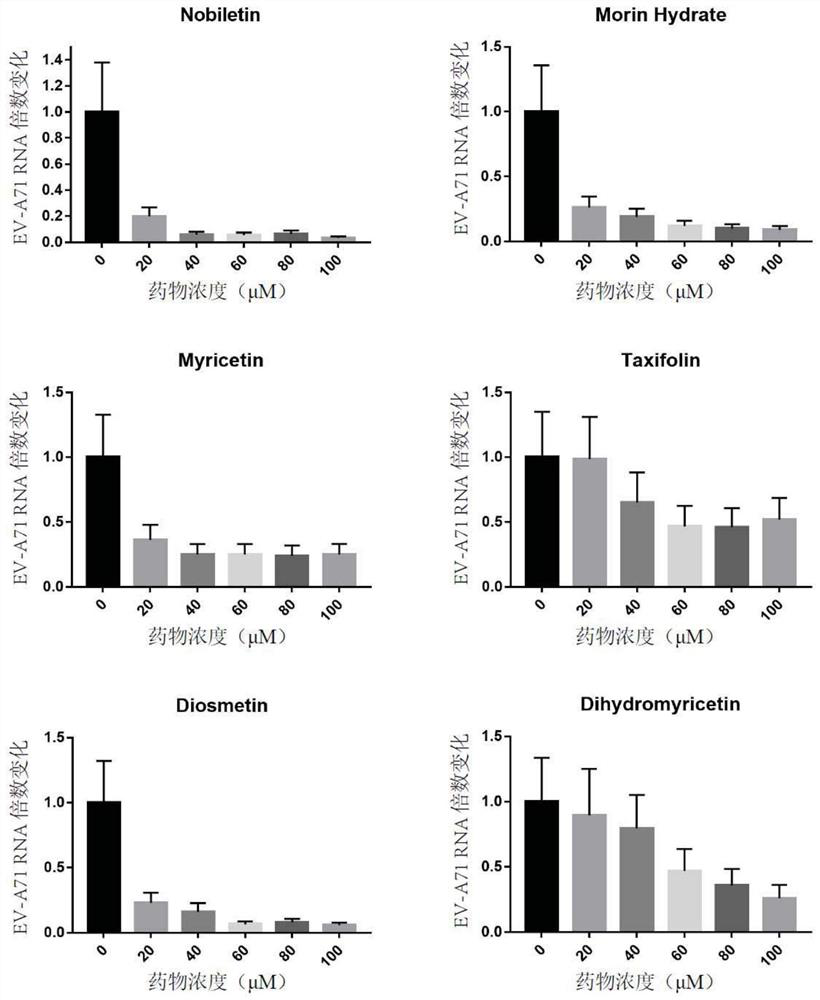
[0135] Inhibition to EV-A71 virus in embodiment 2RD cells
[0136] RD cells at 1 x 10 5 cells / well were seeded in a 24-well plate. 37°C, CO 2 Incubate in the incubator until the cells cover the bottom of the plate. Set the titer to 1 x 10 4 PFU / ml of EV-A71 virus liquid GZ203AKL21 was added to each well in an amount of 200 μL per well. 37°C, CO 2 Incubate in an incubator for 1 hour, discard the virus solution in the well, wash each well twice with PBS, and then add 1ml of DMEM culture solution to each well. Then the candidate drug with a concentration of 1 mM was added to the wells in the amounts of 0 μL, 20 μL, 40 μL, 60 μL, 80 μL, and 100 μL, and three replicate wells were set for each concentration. 37°C, CO 2 After incubation in the incubator for 24 hours, 50 μL of the supernatant was added to the virus RNA extraction kit to start RNA extraction. A reverse transcription kit was used for RNA to generate cDNA, which was used as a template for quantitative PCR amplifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com