Preparation method of spontaneous dispersing single atom Ag1/Co3O4 catalyst
A spontaneous dispersion and catalyst technology, applied in catalyst activation/preparation, physical/chemical process catalysts, separation methods, etc., can solve problems affecting catalyst performance, precious metals are difficult to disperse, precious metals are easy to agglomerate, etc., to achieve excellent catalytic performance and stability Well, overcome the effect of reunion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
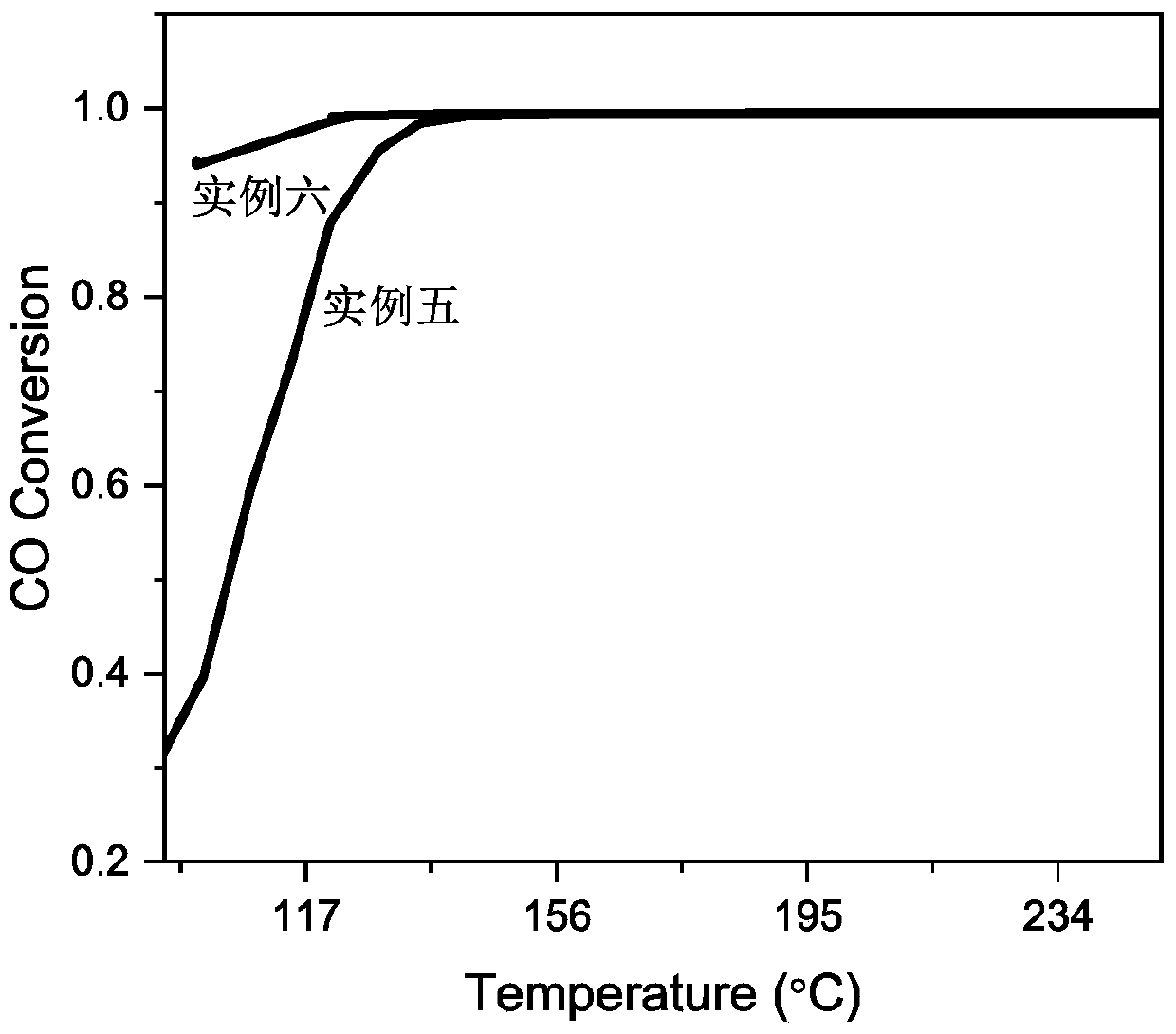
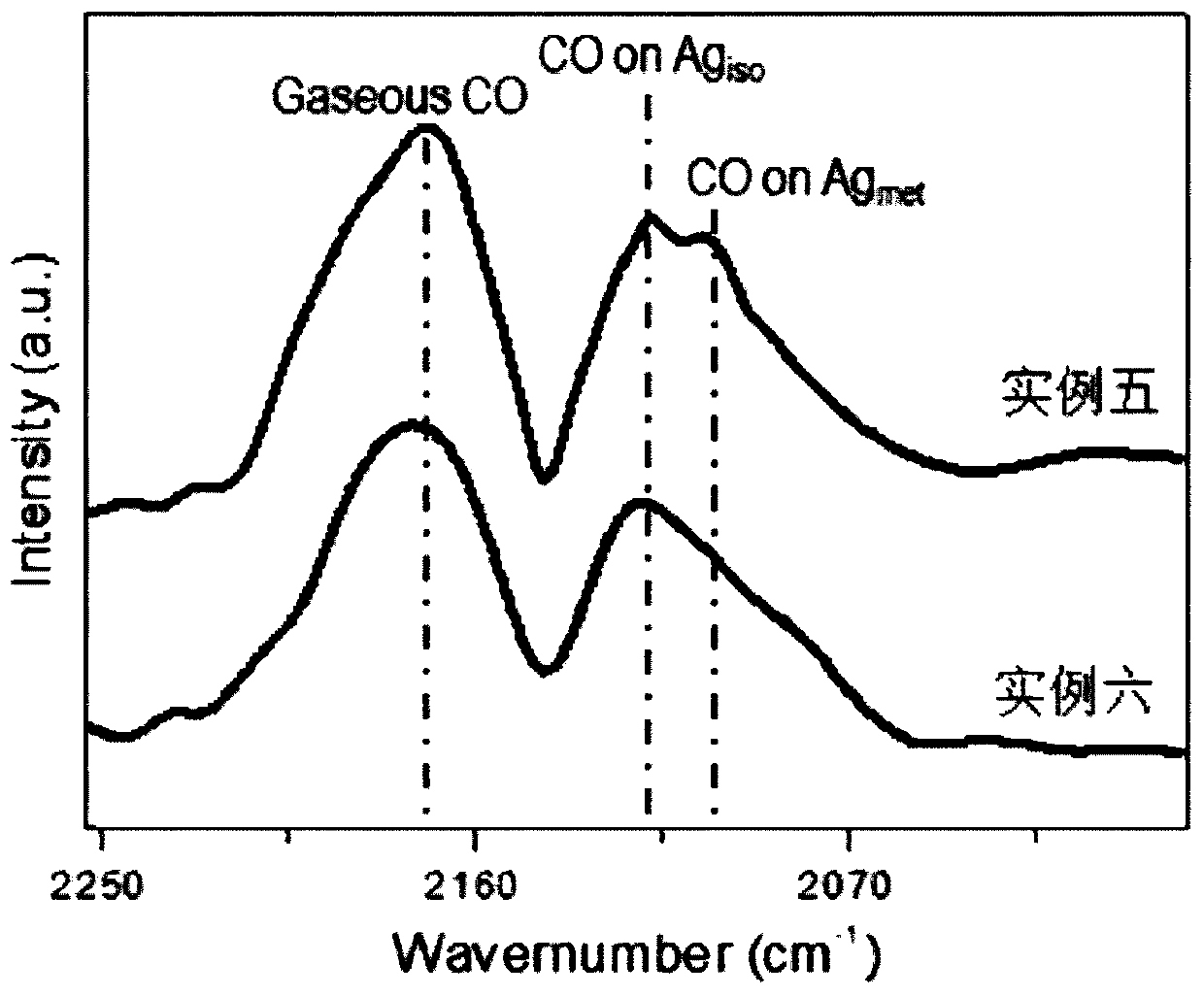
Examples
Embodiment 1
[0028] (1) Take 12g Co(AC) 2 4H 2 O was dissolved in 600mL of ethylene glycol, and the mixture was transferred to a 1L three-necked flask, connected to a condenser for condensation and reflux, heated and stirred at 180°C for 6h, and reacted to obtain cobalt glycolate.
[0029] (2) Centrifuge the solid obtained in step (1), wash with ethanol 3-5 times to remove ethylene glycol, and dry at 80° C. for 12 hours.
[0030] (3) Dissolve 0.44g of silver nitrate in 10mL of deionized water, add ammonia water dropwise until precipitation occurs, continue to add ammonia water dropwise until the solution is clear, and then continue to drop 2mL of excess ammonia water.
[0031] (4) Take 2 g of the solid obtained in step (2), ultrasonically disperse it in 20 mL of deionized water, and slowly add H 2 o 2 solution, prepared Co with exposed {110} and {100} facets 3 o 4 carrier.
[0032] (5) Add step (3) solution and H to the solution in step (4) dropwise 2 o 2 solution, the rate of addi...
Embodiment 2
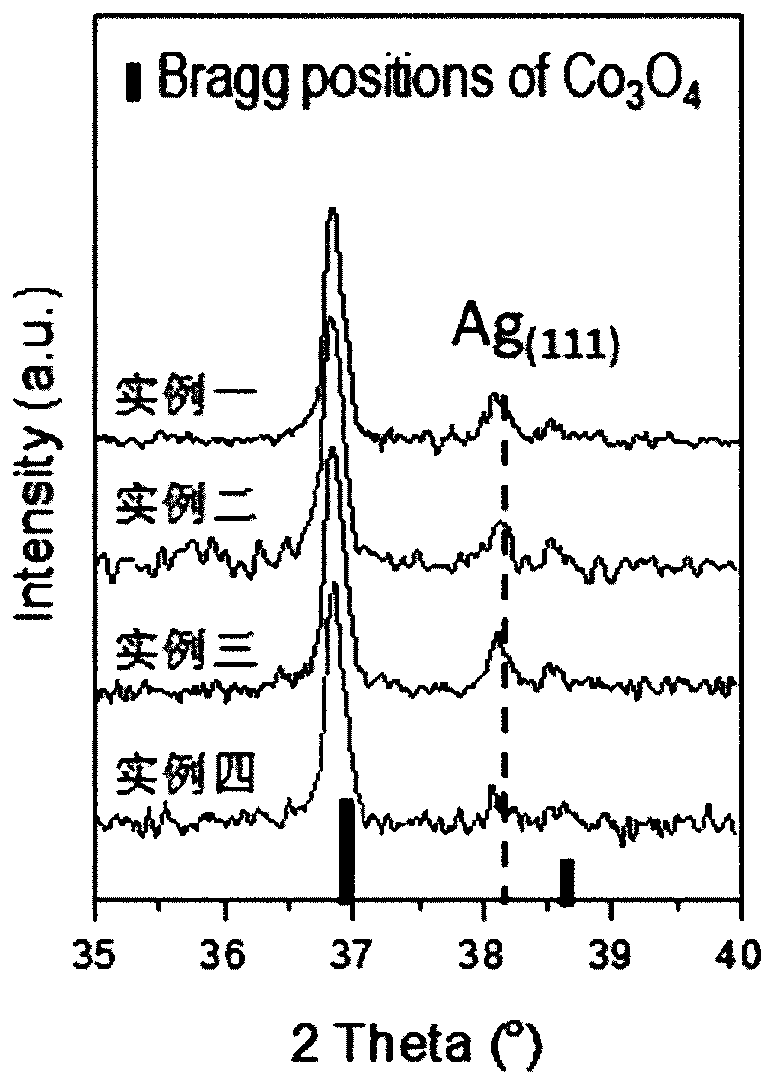
[0036] The difference between this example and Example 1 is that the catalyst obtained in step (6) in Example 1 was heat-treated in an air atmosphere at 150° C. for 30 minutes. In order to verify the success of the prepared catalyst and the Ag state on the surface of the Ag catalyst, X-ray powder diffraction analysis was carried out on the catalyst, the results are as follows figure 1 .
Embodiment 3
[0038]The difference between this example and Example 1 is that the catalyst obtained in step (6) in Example 1 was heat-treated in an air atmosphere at 200° C. for 30 minutes. In order to verify the success of the prepared catalyst and the Ag state on the surface of the Ag catalyst, X-ray powder diffraction analysis was carried out on the catalyst, the results are as follows figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com