Patents
Literature
155 results about "Lattice oxygen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
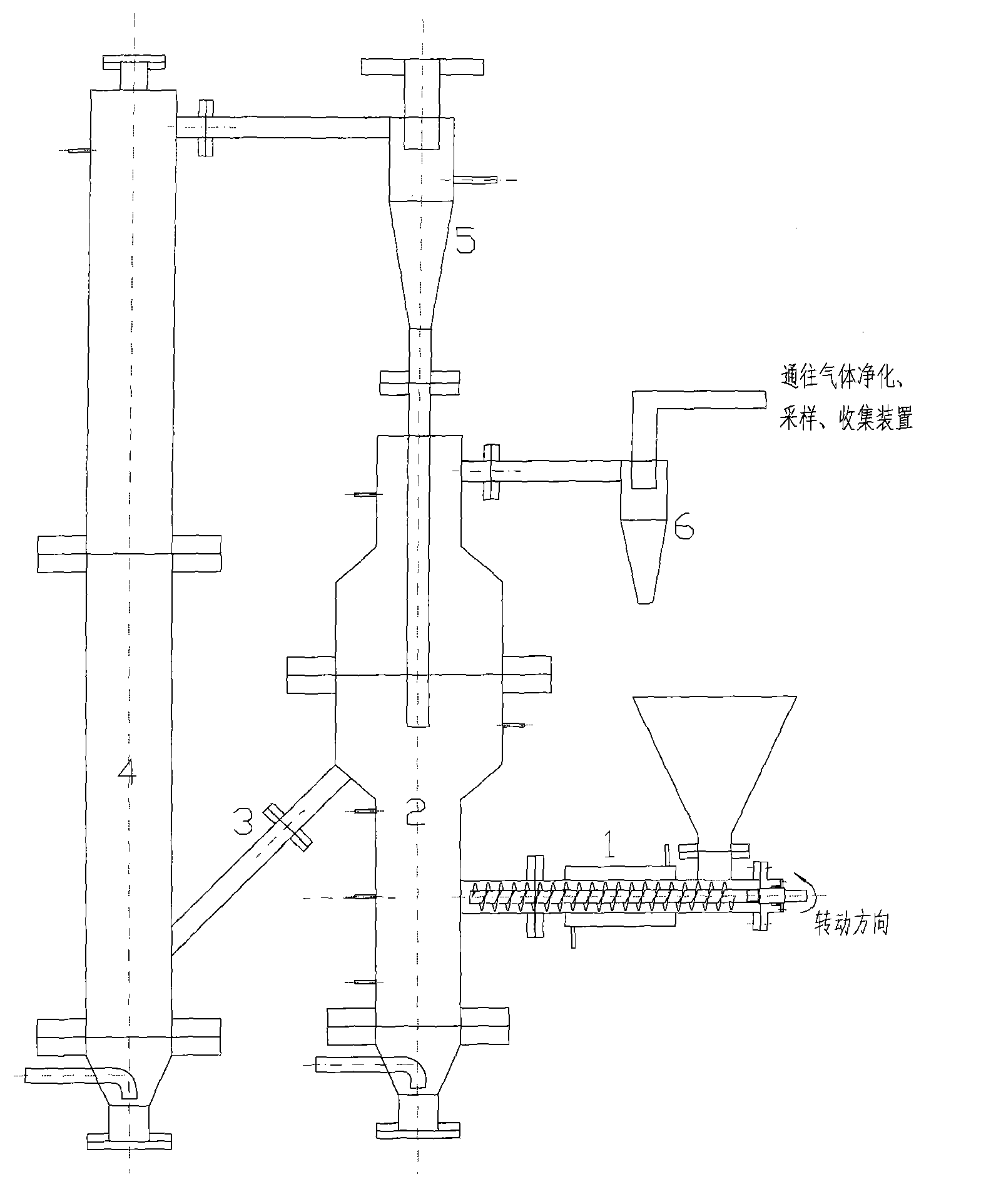
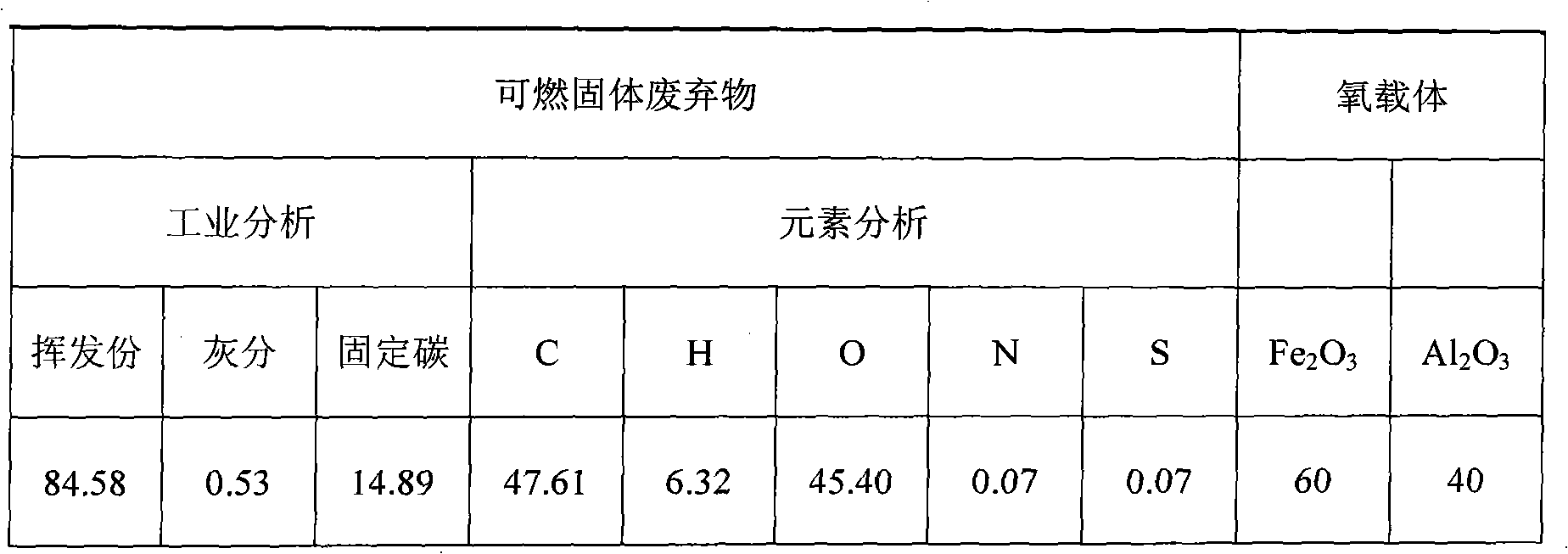
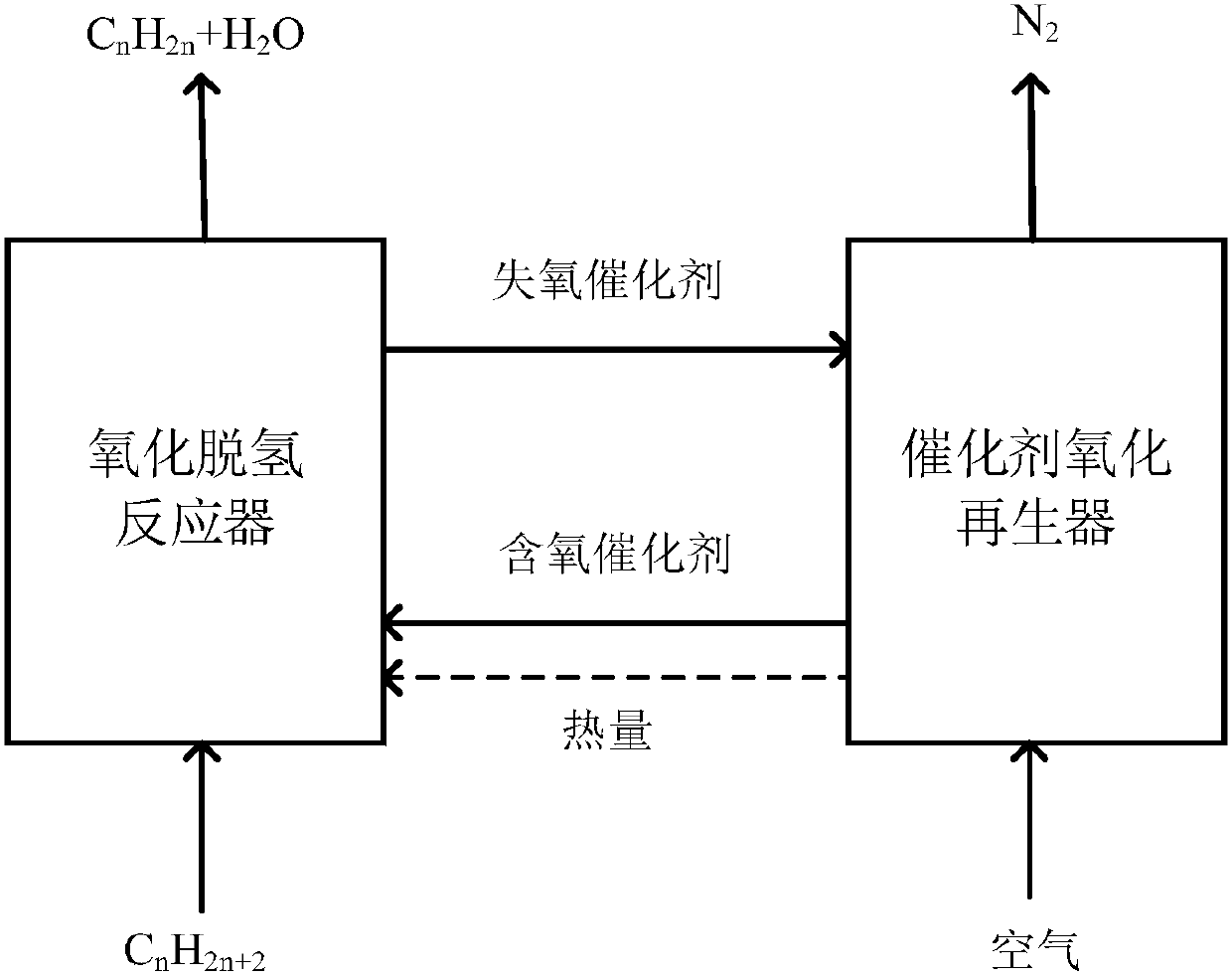
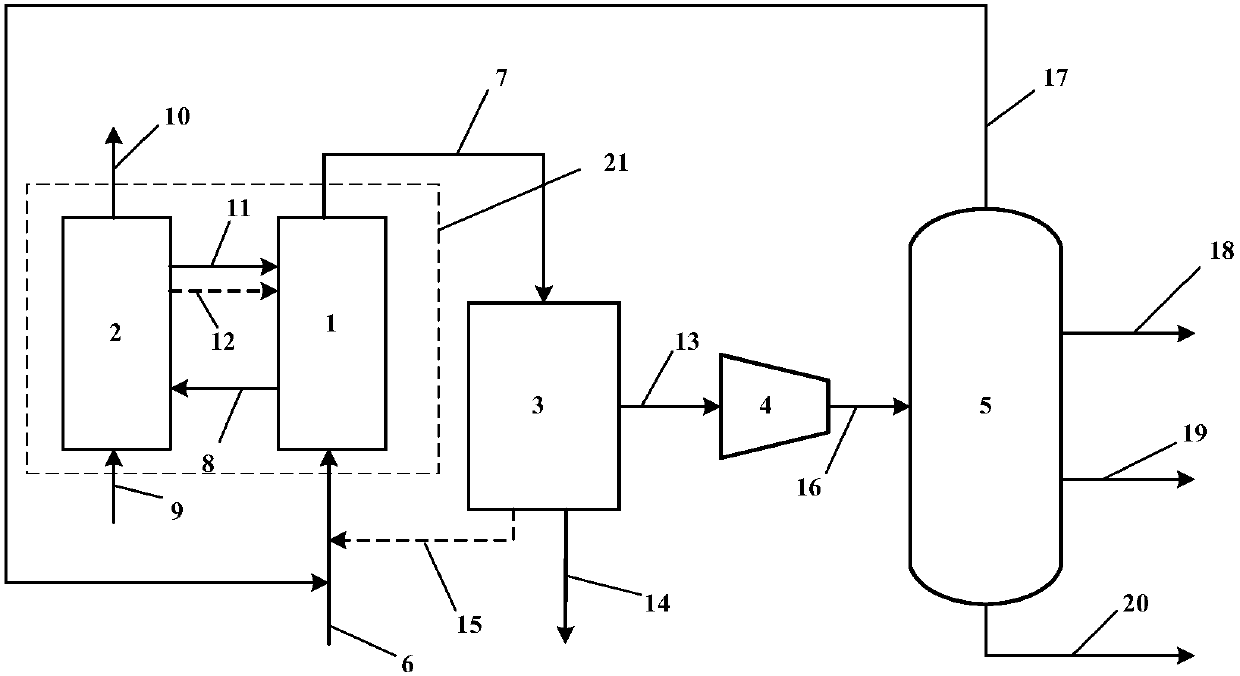
Method for producing synthesis gas by combustible solid waste chemical chain gasification and interconnected fluidized bed reactor
ActiveCN101638590ALower Gasification CostsHigh calorific valueCombined combustion mitigationChemical/physical processesFluidized bedLattice oxygen
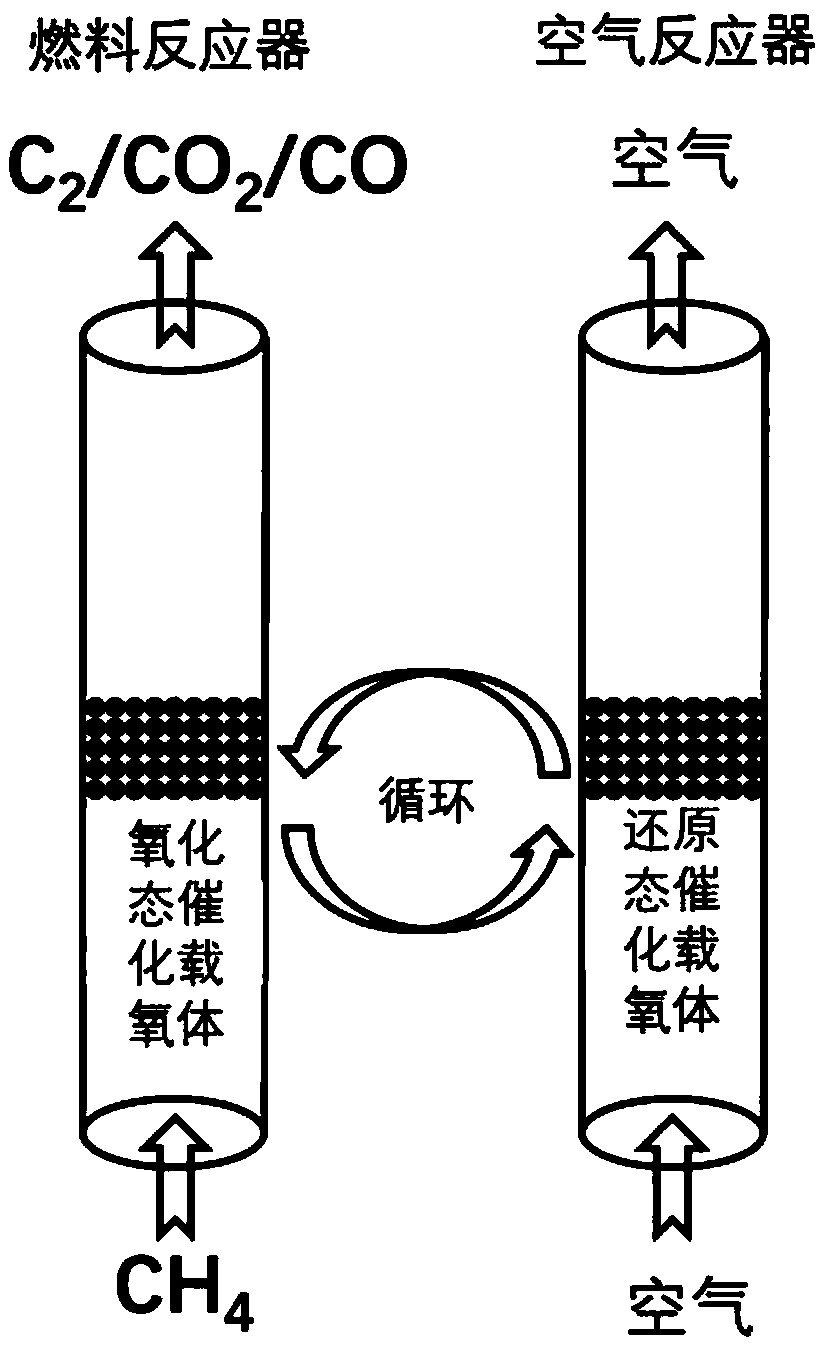
The invention provides a method for producing synthesis gas by combustible solid waste chemical chain gasification and an interconnected fluidized bed reactor. The method is implemented in an interconnected fluidized bed reactor, and the interconnected fluidized bed reactor comprises a fuel reactor and an air reactor communicated with each other internally. The combustible solid waste produces gasification reaction with a carrier of oxygen to produce synthesis gas, and the oxygen element necessary for the gasification of combustible solid waste comes from the lattice oxygen in the carrier component of oxygen; the carrier of oxygen without lattice oxygen is transported to the air reactor, in the air reactor, the carrier of oxygen is re-oxidized by high-temperature air to recovery to latticeoxygen; the carrier of oxygen recovered with lattice oxygen is carried out the air reactor by high-speed air, returns back to the fuel reactor for cycle use and produces gasification reaction with the combustible solid waste again. The invention relates to a high-efficiency combustible solid waste gasification technique breaking the prior art, and the invention can produce high-quality synthesisgas and reduce the contents of tar and carbon deposite during the gasification process.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
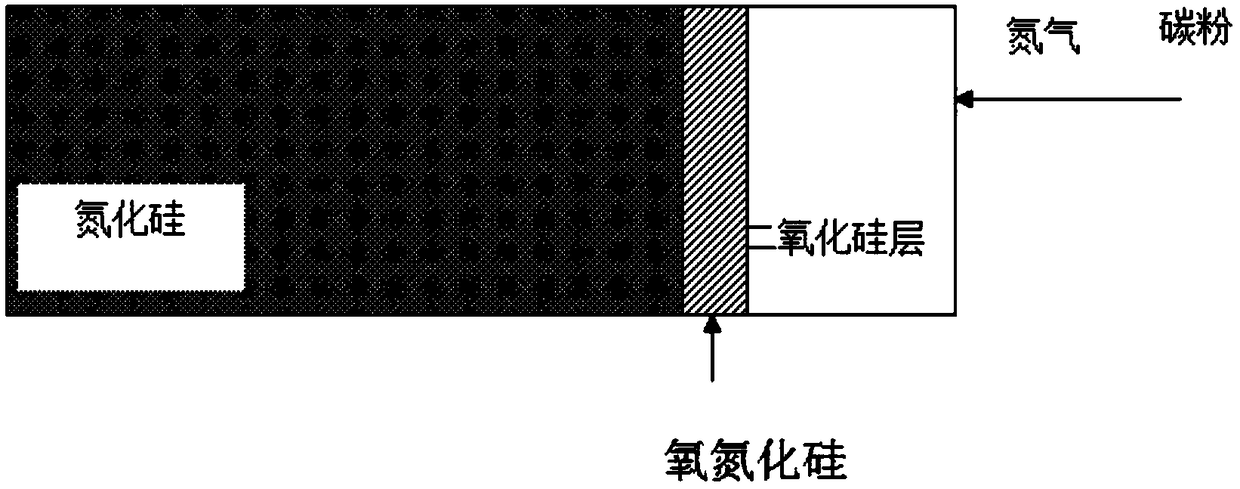
High-heat-conductivity silicon nitride ceramic and preparation method thereof
The invention provides high-heat-conductivity silicon nitride ceramic and a preparation method thereof, which are used for solving the technical problem that the existing heat conductivity is low. Thepreparation method comprises the following steps: performing deoxygenation treatment on silicon nitride powder, naturally cooling the silicon nitride powder, and grinding and sieving the obtained silicon nitride powder; mixing the powder and a sintering aid under the action of a mixed medium, and drying and sieving after mixing to obtain powder; performing pressing formation to obtain a silicon nitride ceramic green body; and performing gas pressure sintering to obtain the silicon nitride ceramic material. Compared with the prior art, the high-heat-conductivity silicon nitride ceramic and thepreparation method thereof have the following advantages: the silicon nitride powder is subjected to deoxygenation treatment, the oxygen content of the original powder is low, the degree of reducingthe lattice oxygen content in the sintering process is higher, and phonon scattering is avoided, so that the heat conductivity of the silicon nitride ceramic is improved; and the prepared silicon nitride ceramic has high heat conductivity, high thermal shock resistance and high-temperature resistance, is safe to use and is a silicon nitride ceramic substrate material with excellent mechanical, thermal and electric comprehensive performance.
Owner:HARBIN INST OF TECH
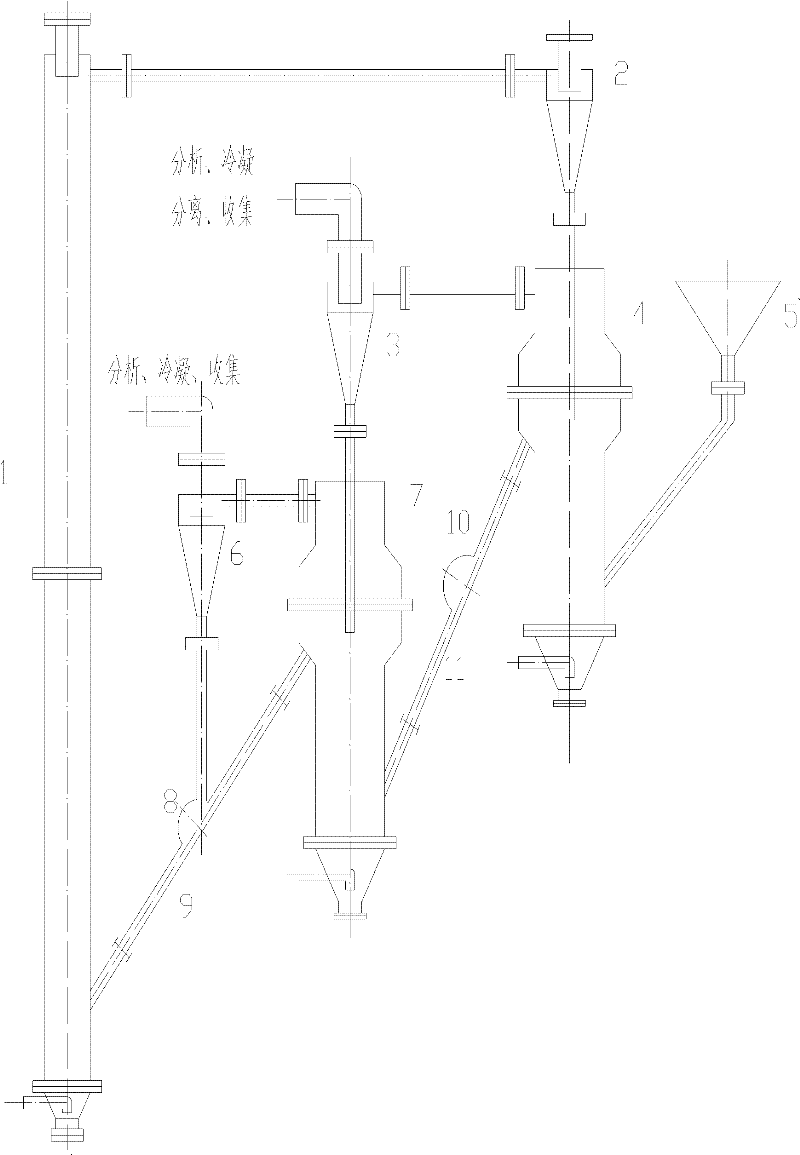
Method and device for producing hydrogen by using chemical chain
InactiveCN102198934ARealize automatic separationAvoid emissionsCarbon compoundsHydrogen productionHydrogenCombustion
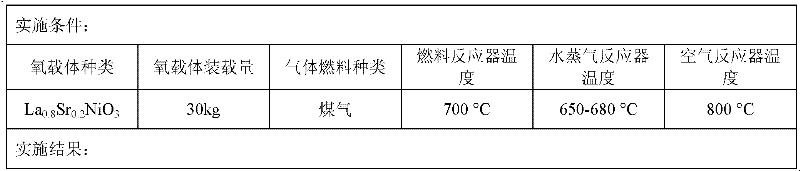
The invention provides a method and device for producing hydrogen by using a chemical chain. In the method, perovskite type oxide is selected to serve as an oxygen carrier; a main body structure of the device comprises a fuel reactor, a water vapor reactor and an air reactor. Fuel and the oxygen carrier undergo a chemical chain combustion reaction in the fuel reactor to generate CO2 and H2O; oxygen elements required by the fuel are from lattice oxygen of the oxygen carrier; the reduced oxygen carrier is delivered to the water vapor reactor to react with high-temperature water vapor, restore part of lattice oxygen and simultaneously generate H2; the oxygen carrier is delivered to the air reactor to react with high-temperature air to be further oxidized and completely restore the lattice oxygen; the oxygen carrier is carried to a cyclone separator by high-speed air current; gas is emptied; and the oxygen carrier is delivered again to the fuel reactor to be recycled. According to the method and device, the automatic separation of CO2 is realized when pure H3 is produced and the emission of greenhouse gas is avoided. The oxygen carrier has the advantages of sable structure, favorable oxygen loss and supply capabilities and long service life.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
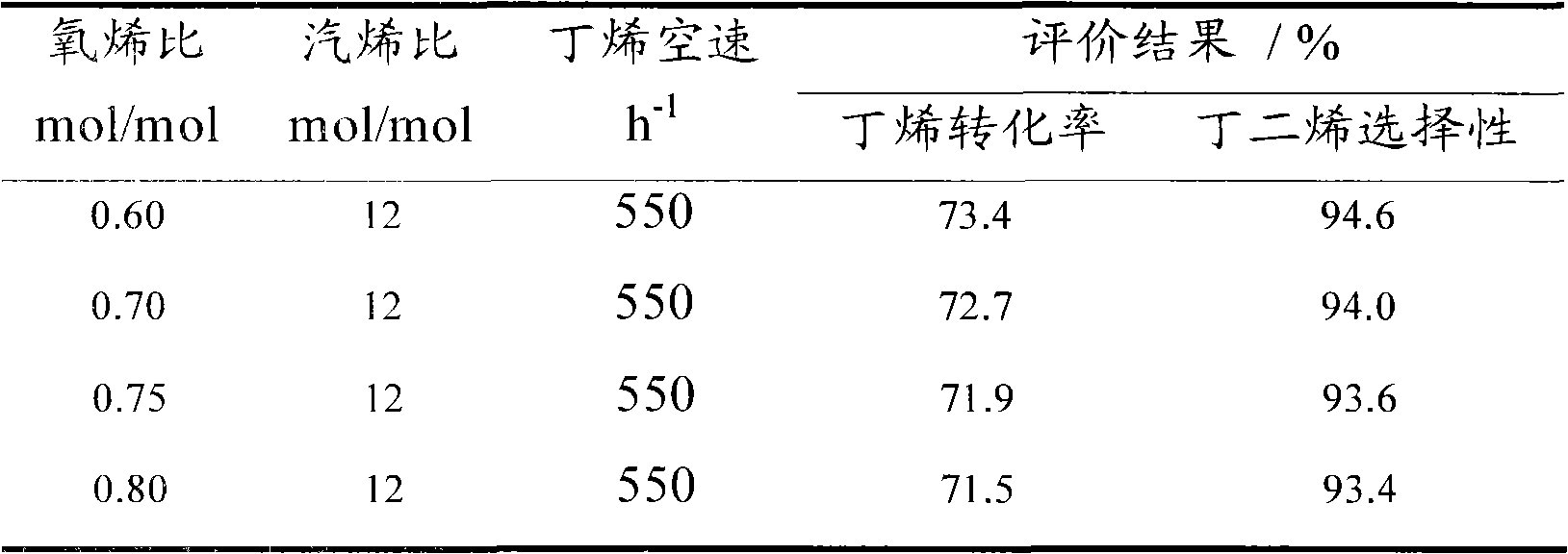
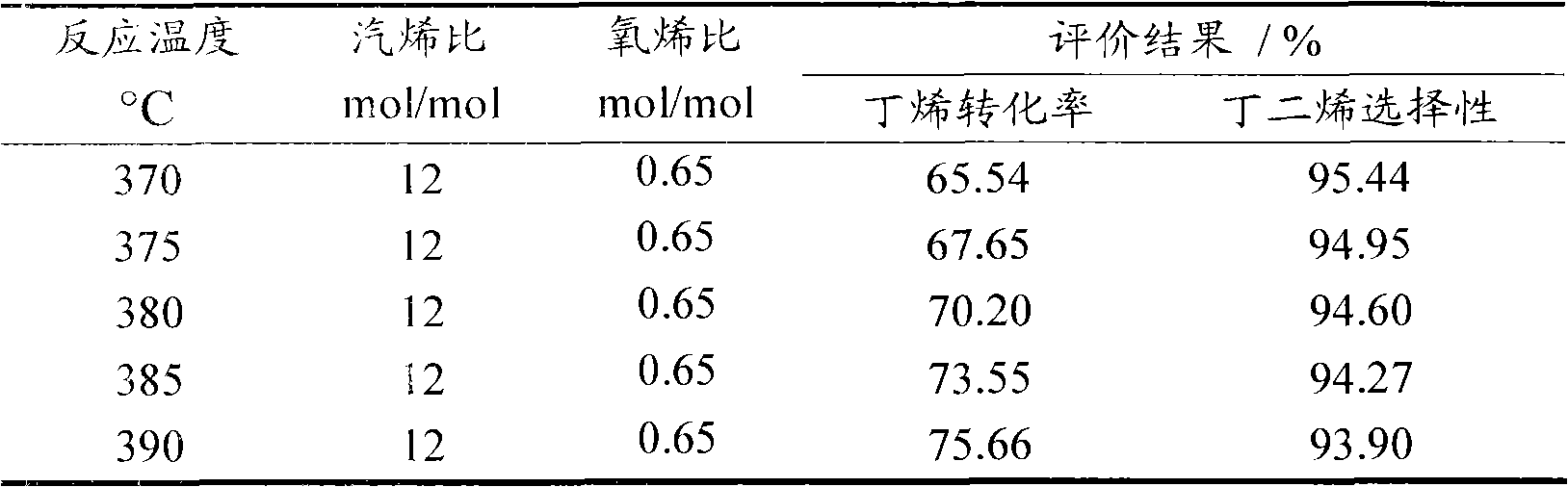
Method for producing butadiene by oxidatively dehydrogenating butene and used catalyst
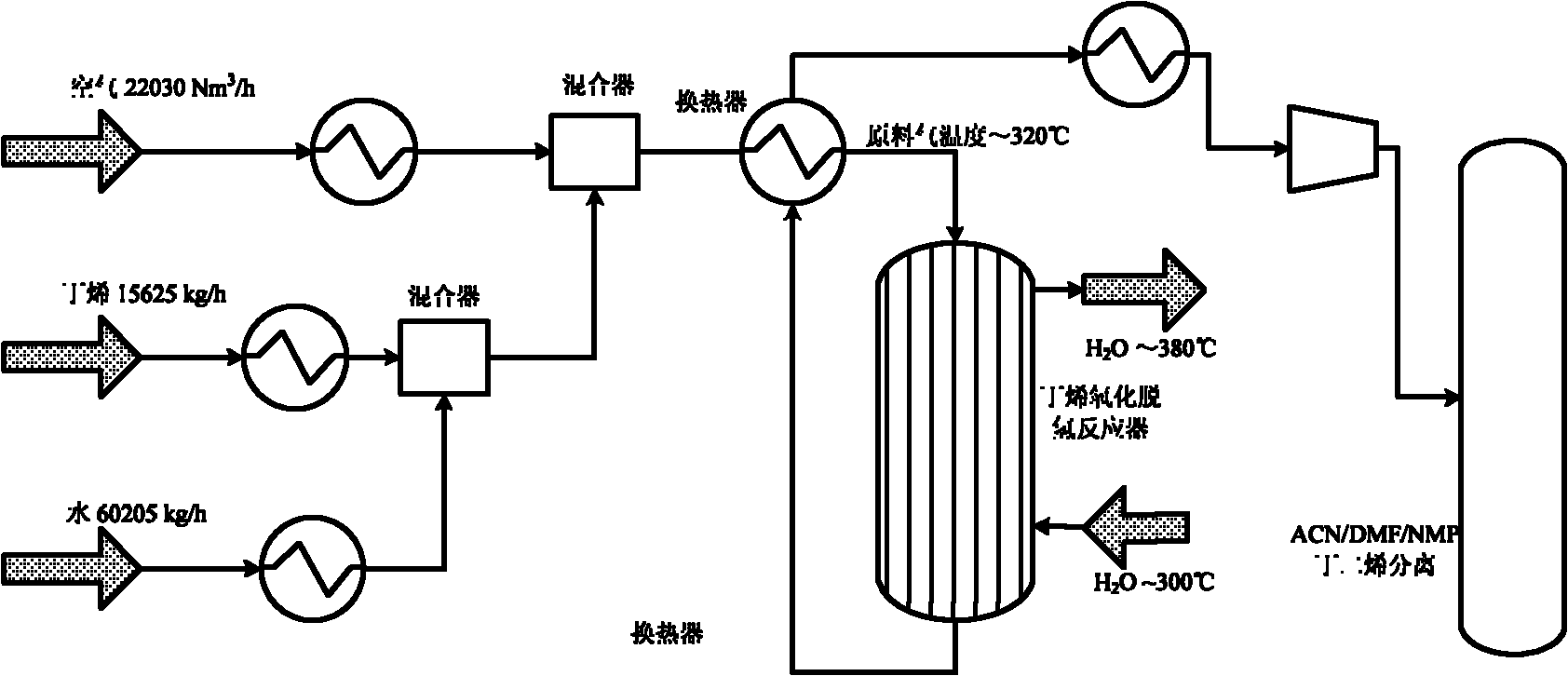
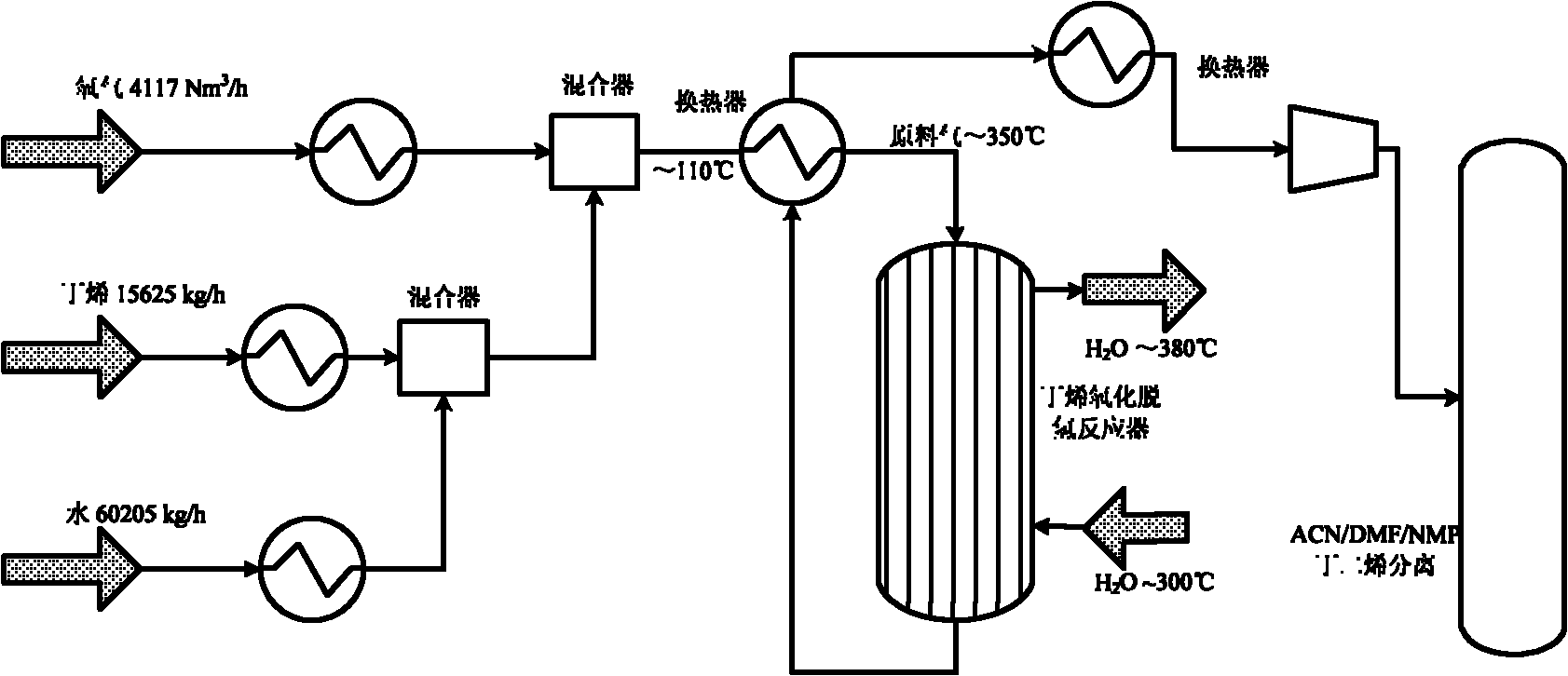
ActiveCN103102238AHigh yieldHigh selectivityHydrocarbonsMetal/metal-oxides/metal-hydroxide catalystsExtractive distillationLattice oxygen
The invention discloses a method for producing butadiene by oxidatively dehydrogenating butane and a used catalyst. The method comprises the following steps of using product gas heat and reaction heat to exchange heat with raw material butene, water, oxygen or air, and then catalytically converting on a fixed bed to effectively prepare butadiene. The invention in particular relates to an alpha-Fe2O3, ZnFe2O4 composite oxide lattice oxygen catalyst. The raw material gas preheated to a certain temperature is heated to the temperature for reaction by adopting the sensible heat of the reaction product gas, the temperature of the reactor is controlled by steam heat-exchange, the raw material gas is heated by the sensible heat of the product gas and the stable operation of the reactor is realized, the oxidatively dehydrogenated rough butadiene logistics generated gas is separated through the processes of compression, oil-absorption and de-absorption, and extractive distillation of a butadiene solvent so as to efficiently, continuously and stably prepare the butadiene product, the once through yield of the butadiene is more than 80%.
Owner:CHINA PETROLEUM & CHEM CORP
Vanadium-phosphorus-oxide (VPO) catalyst and application in preparation of crylic acid (ester) by reacting acetic acid (ester) with formaldehyde
InactiveCN103816930AHigh catalytic efficiencyHigh activityPhysical/chemical process catalystsOrganic compound preparationO-Phosphoric AcidGeneration rate
The invention discloses a vanadium-phosphorus-oxide (VPO) catalyst for preparing crylic acid or methyl acrylate by condensing acetic acid or methyl acetate with formaldehyde. A preparation method of the vanadium-phosphorus-oxide (VPO) catalyst comprises the following steps: reducing pentavalent vanadium (vanadium pentoxide) through monobenzyl alcohol or a mixed alcohol of benzyl alcohol / isobutyl alcohol, adding a polyethylene glycol (PEG6000) surfactant into an alcohol medium, then adding phosphoric acid, adjusting a ratio of P (phosphorus) / V (vanadium) to be 1.05 to prepare a catalyst precursor, and activating the catalyst precursor in pure nitrogen, pure air and 1.5%( volume fraction) butane-air mixture atmosphere. Since the PEG is added and / or the types of prepared medium alcohols and different precursor activation atmospheres are changed in the preparation process of the catalyst, a crystal phase shape and crystallinity, reaction reactivity of lattice oxygen, ratio of V<5+> / V<4+> on the surface of the catalyst can be significantly modulated, thus significantly modulating reaction behavior of the catalyst. The catalyst for preparing crylic acid (ester) by condensing acetic acid (methyl ester) with formaldehyde is high in catalytic efficiency, and by-products are few; a greatest generation rate of (crylic acid plus acrylic ester) can reach 32.1micromoles / gcat<-1> / min<-1>.
Owner:NANJING UNIV
Lattice oxygen catalyst used for preparing butadiene through butylene oxidative dehydrogenation, and preparation method thereof
The invention relates to a lattice oxygen catalyst used for preparing butadiene through butylene oxidative dehydrogenation. The catalyst is composed of 80.0-95.0wt% of a substrate comprising alpha-Fe2O3 and MFe2O4, 4.0-19.0wt% of a structural auxiliary agent comprising SiO2 and / or Al2O3, and 0.001-1.0 wt% of a reaction performance modulation auxiliary agent AOx. In the substrate, the content of alpha-Fe2O3 is 10.0-60.0wt%. M is Zn or a composition of Zn and one or more metals selected from alkaline earth metals and transition metals. A is selected from alkali metals, alkaline earth metals, rare earth metals, and compositions thereof. x is 1-2. The invention also relates to a preparation method of the lattice oxygen catalyst used for preparing butadiene through butylene oxidative dehydrogenation.
Owner:北京中石润达科技发展有限公司
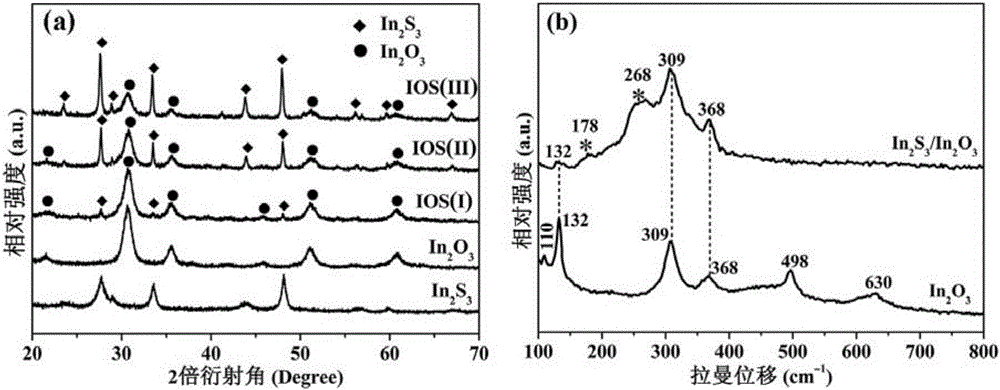
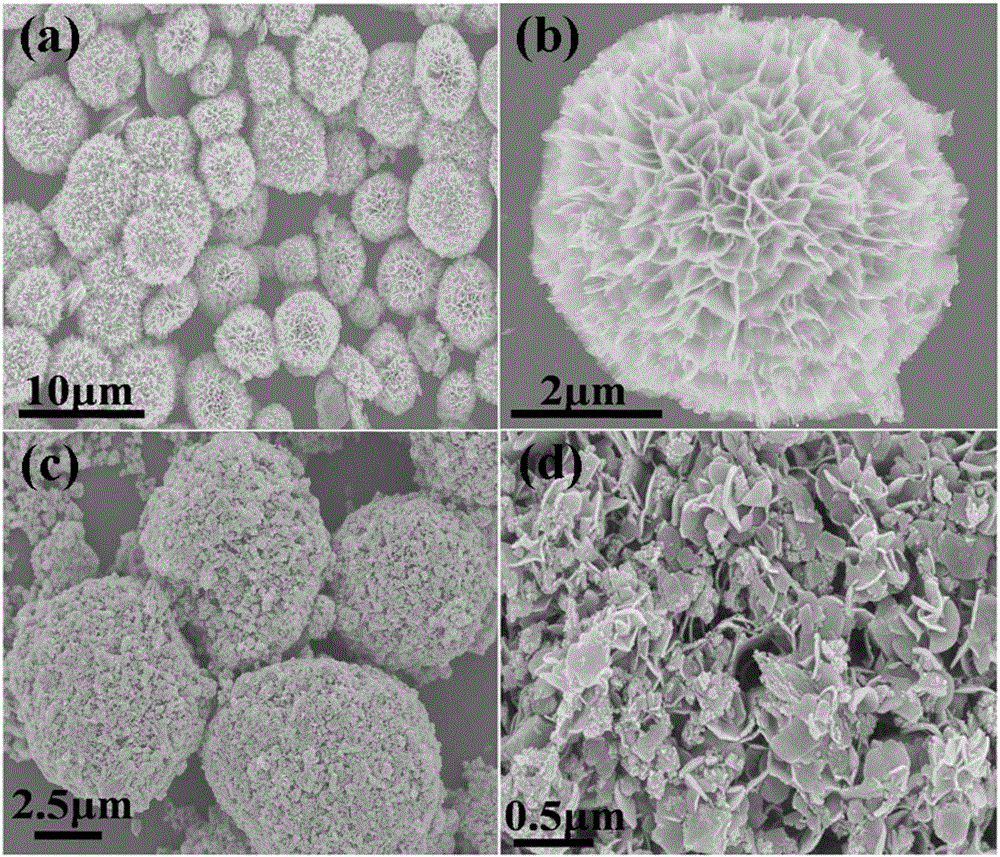
Three-dimensional flower-like In2S3/In2O3 composite microsphere photocatalytic material and preparation method thereof
InactiveCN105664973AIncrease profitInhibitory complexGas treatmentPhysical/chemical process catalystsHeterojunctionLattice oxygen
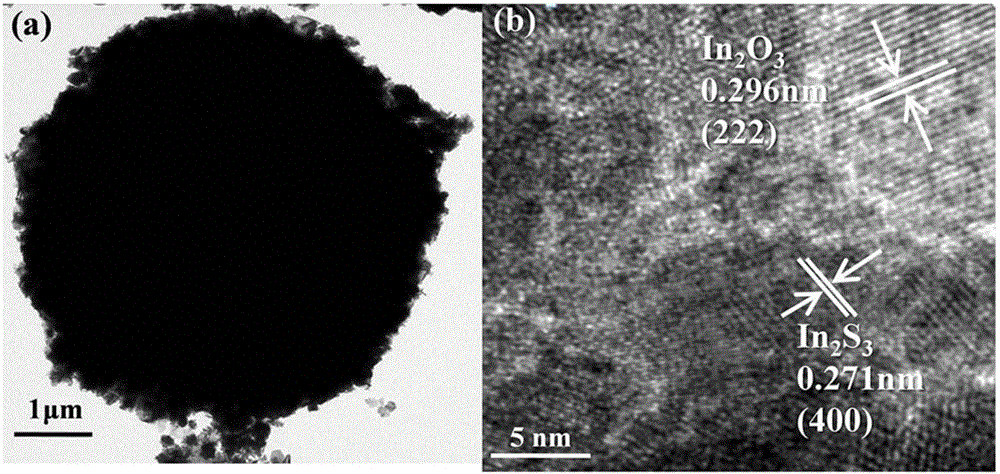
The invention discloses a three-dimensional flower-like In2S3 / In2O3 composite microsphere photocatalytic material and a preparation method thereof, belonging to the field of pollution control and technology. The preparation method comprises the following steps of taking thioacetamide as a sulfur source, substituting lattice oxygen in the In2O3 with sulfur ions through an ion exchange reaction, performing in situ generation of an In2S3 nanosheet on the surface of flower-like In2O3, and forming a heterojunction structure, wherein the morphology of the three-dimensional flower-like In2O3 is uniform, and the particle size of the three-dimensional flower-like In2O3 is 3.0 to 6.5 microns; coating the surface of / In2O3 microflowers with the In2S3 nanosheet. The used raw materials are low in price and easily-obtained, reaction conditions are easily controlled and the requirement on equipment is low, so the preparation method disclosed by the invention is an environmental-friendly preparation method. By using In2S3 / In2O3 composite microspheres, the utilization rate of the In2O3 to visible light can be improved, and the compounding of photon-generated carriers is inhibited; the In2S3 / In2O3 composite microspheres have good application value and prospect in the field of volatile organic contaminants.
Owner:DALIAN UNIV OF TECH
Calcium-titanium-ore type composite oxide La1-xSrxMO3-0.5 beta F beta
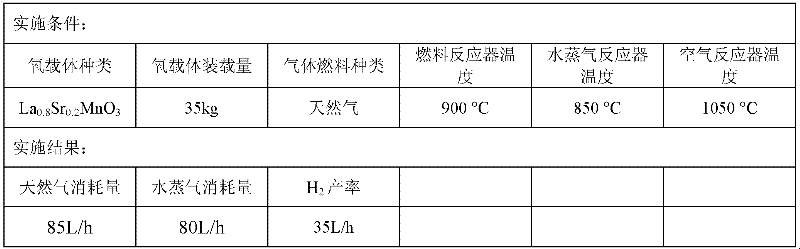
InactiveCN101069844ASimple structureImprove stabilityHydrogenCatalyst activation/preparationLattice oxygenReaction temperature
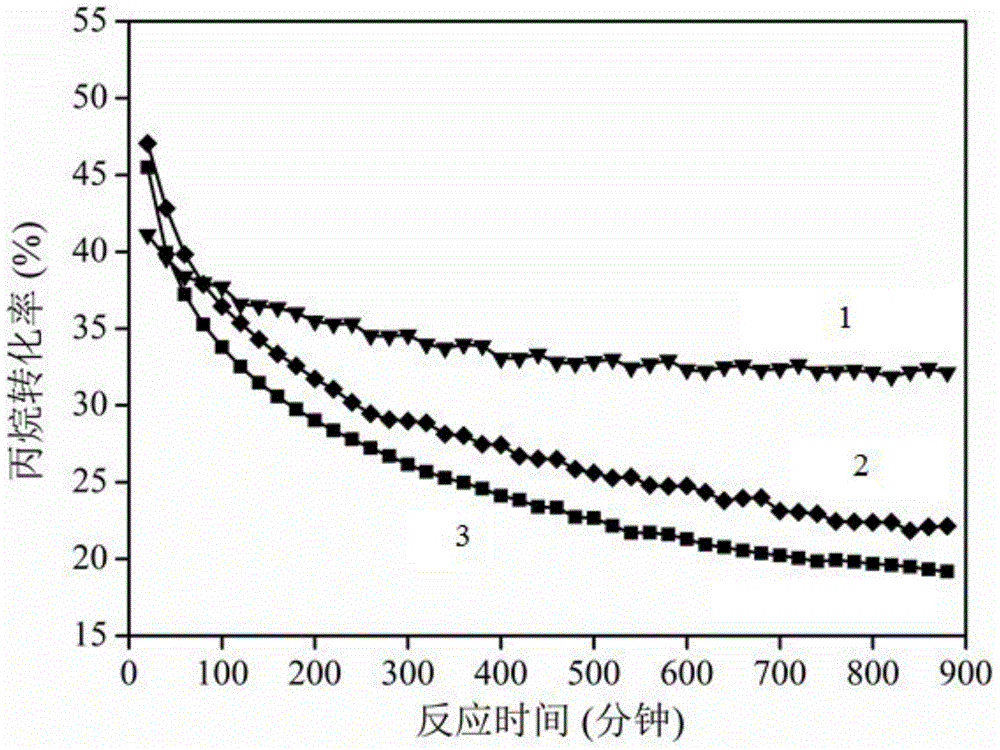
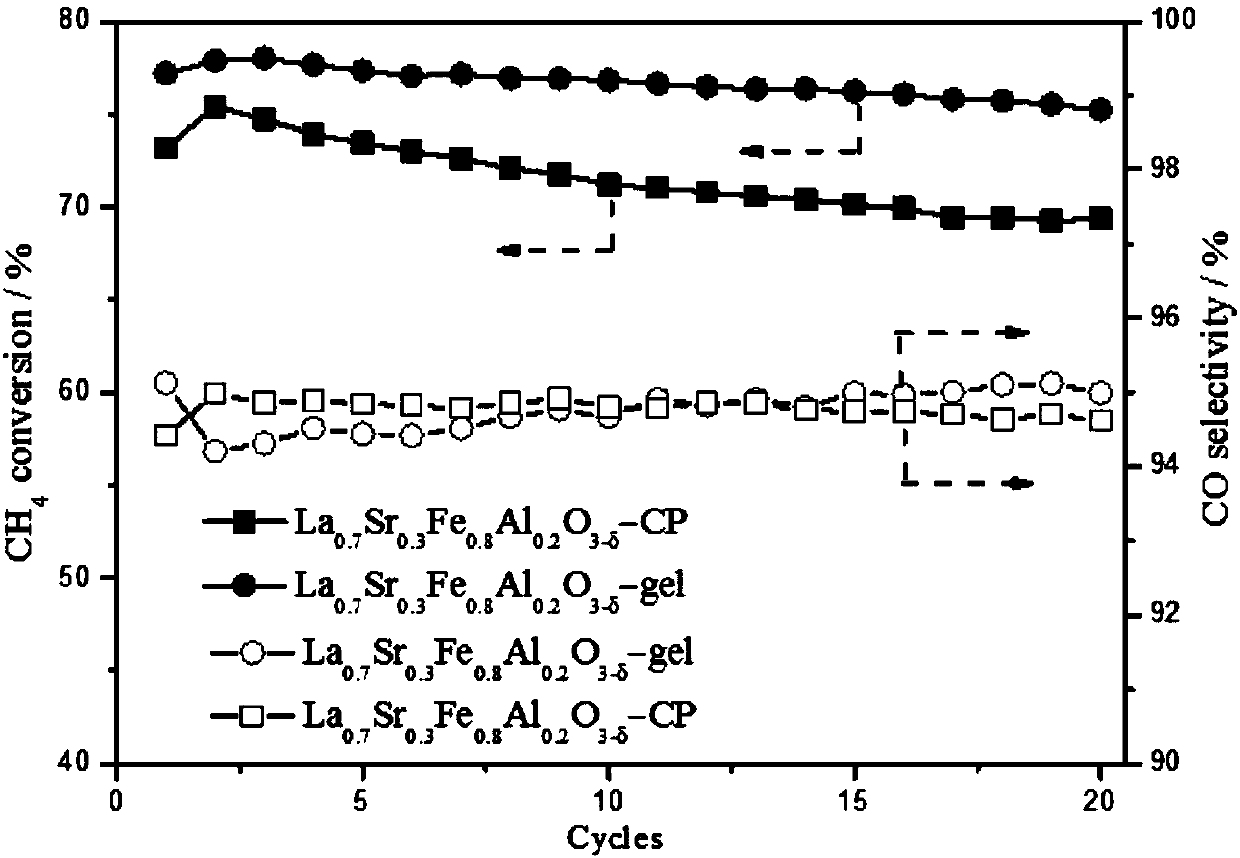
The present invention relates to a kind of La1-x Srx MO3-0.5 beta F beta composite oxide, in which M-Mn or Fe or Ni, x=0-0.4, beta=0-0.1, x and beta are not zero at the same time. It is mainly formed from perovskite composite oxide, the phase composition is directly related to Sr content in the sample, and its specific surface area is 4.5-13.4 sq.m / g. The invented La1-x SrxMO3-0.5 beta F beta composite oxide can be used as an oxygen carrier, its lattice oxygen can be utilized for directly oxidate methane to prepare synthetic gas. In the reaction temperature range of 800-900deg.C its CH4 conversion rate is 15-55%, CO selectivity is 5-99%, and H2 / CO (mol / mol)=1.9-10.5:1.0. Said invention also discloses its preparation method.
Owner:NANJING UNIV
Catalyst for preparing butadiene through butylene oxydehydrogenation and use thereof
ActiveCN105521796AHigh selectivityImprove stabilityHydrocarbonsMetal/metal-oxides/metal-hydroxide catalystsButadiene oxideLattice oxygen
The invention relates to a catalyst for preparing butadiene through butylene oxydehydrogenation and a use thereof. The catalyst mainly solves the problem that the existing catalyst for preparing butadiene through butylene oxydehydrogenation needs separation of butane in butylene raw materials and has different conversion rates of three isomers such as 1-butene, cis-2-butene and trans-2-butene under the same reaction conditions so that a butadiene yield is low. The lattice oxygen catalyst comprises alpha-Fe2O3, ZnFe2O4, MgFe2O4 and one or more M metal oxides and M represents one or more of Cr, Mn, V, Sb, Ce, Ga and In. The catalyst realizes efficient, stable and continuous preparation of a butadiene product and can be used for industrial production for preparing butadiene through butylene oxydehydrogenation.
Owner:CHINA PETROLEUM & CHEM CORP +1
Platinum-gallium catalyst loaded on double-oxide composite carrier as well as preparation method and application of platinum-gallium catalyst
ActiveCN104525196AHigh selectivityImprove stabilityCatalyst carriersHydrocarbonsAlkaneCarbon storage
The invention discloses a platinum-gallium catalyst loaded on double-oxide composite carrier as well as a preparation method and an application of the platinum-gallium catalyst. A CeO2-Al2O3 double-oxide carrier is prepared by using CeO2-Al2O3 double-oxide as a carrier, Pt as an active component and Ga as an assistant in a leaching method; the obtained carrier is steeped into chloroplatinic acid and gallium nitrate solutions, is dried and baked to obtain the catalyst. The catalyst is suitable for low alkane dehydrogenized olefin under a hydrogen atmosphere. Taking propane dehydrogenized propylene as an example, because of the addition of CeO2, lattice oxygen is provided to help inhibiting carbon storage; alloys are formed by Ga and Pt to change acting force of products and reactants and Pt in activity center, and Ga is embedded into the lattice of CeO2, so that the oxygen storage and oxygen moving capability of CeO2 are improved. Because of the addition of CeO2 and Ga, the selectivity of propylene and the carbon storage resisting capability are improved, and the relatively-high reaction stability of the catalyst under a high-temperature condition is guaranteed.
Owner:TIANJIN UNIV
Fluidized-bed catalyst for preparing unsaturated nitrile by ammoxidation
InactiveCN103418400ACarboxylic acid nitrile preparationOrganic compound preparationFluidized bedActive component
The invention relates to a fluidized-bed catalyst for preparing unsaturated nitrile by ammoxidation, and mainly solves the problems that in the prior art the lattice oxygen content of a catalyst for acrylonitrile is low, deep oxidation of propylene is easy to occur, the acrylonitrile selectivity is low to cause the low yield of the acrylonitrile. According to the fluidized-bed catalyst for preparing the unsaturated nitrile by ammoxidation, through use of a technical scheme, the problems are well solved. The technical scheme is shown as follows: the fluidized-bed catalyst uses silica sol as a carrier, and contains an active component represented by the following general formula: Mo[12]Bi[a]Fe[b]Ni[c]X[d]Y[e]Z[f]K[g]O[x], wherein b / (a+e)=1.0-3.5. The fluidized-bed catalyst can be used in industrial production of preparation of the acrylonitrile by ammoxidation of the propylene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Oxygen carrier applied to chemical looping partial oxidation process for preparing synthesis gas by using methane, and preparation method and application of oxygen carrier
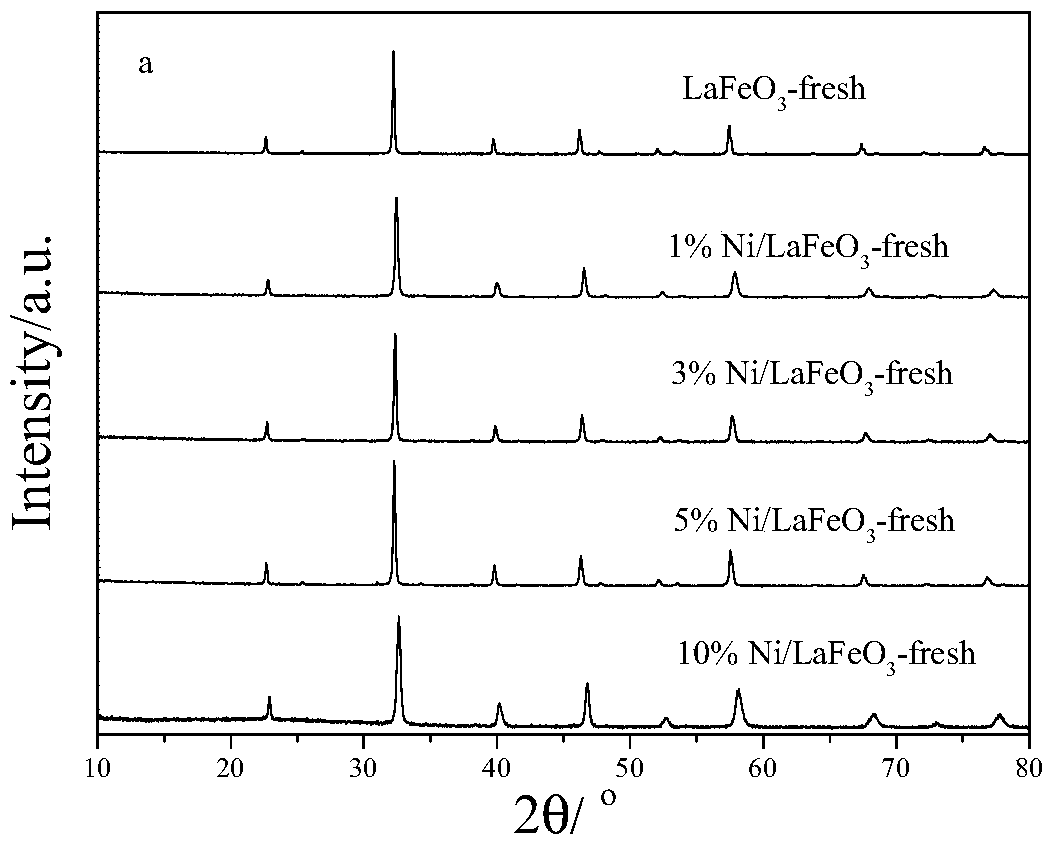
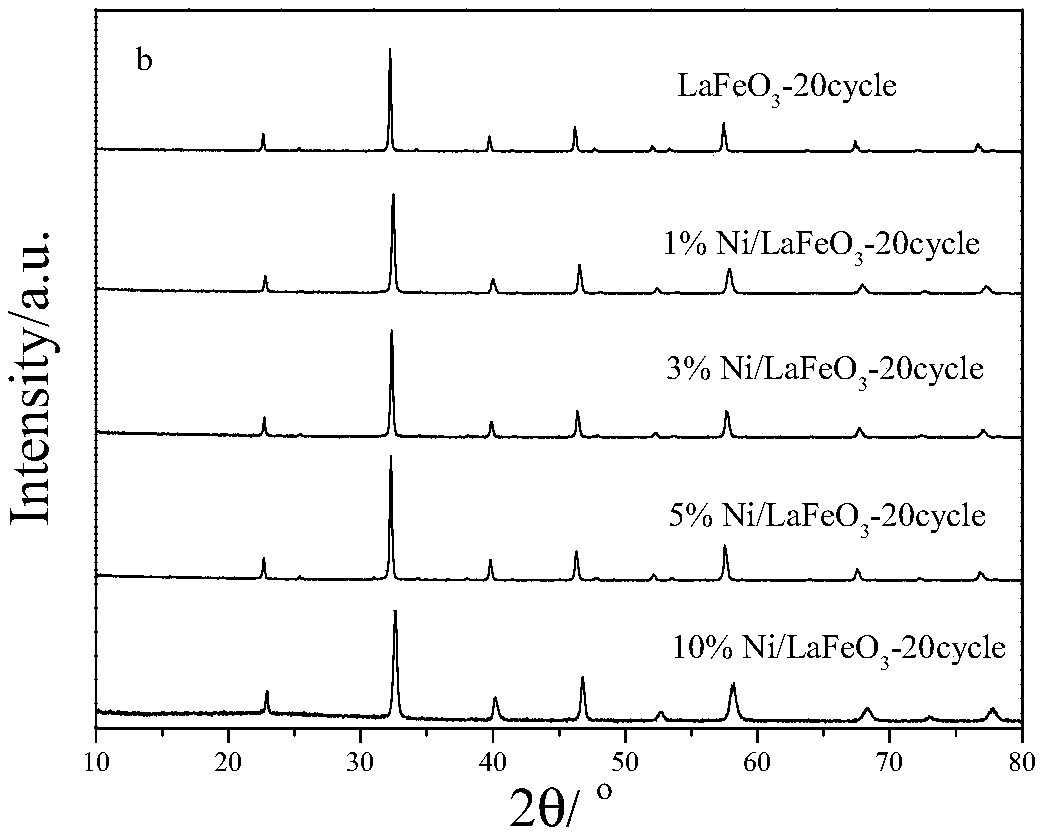
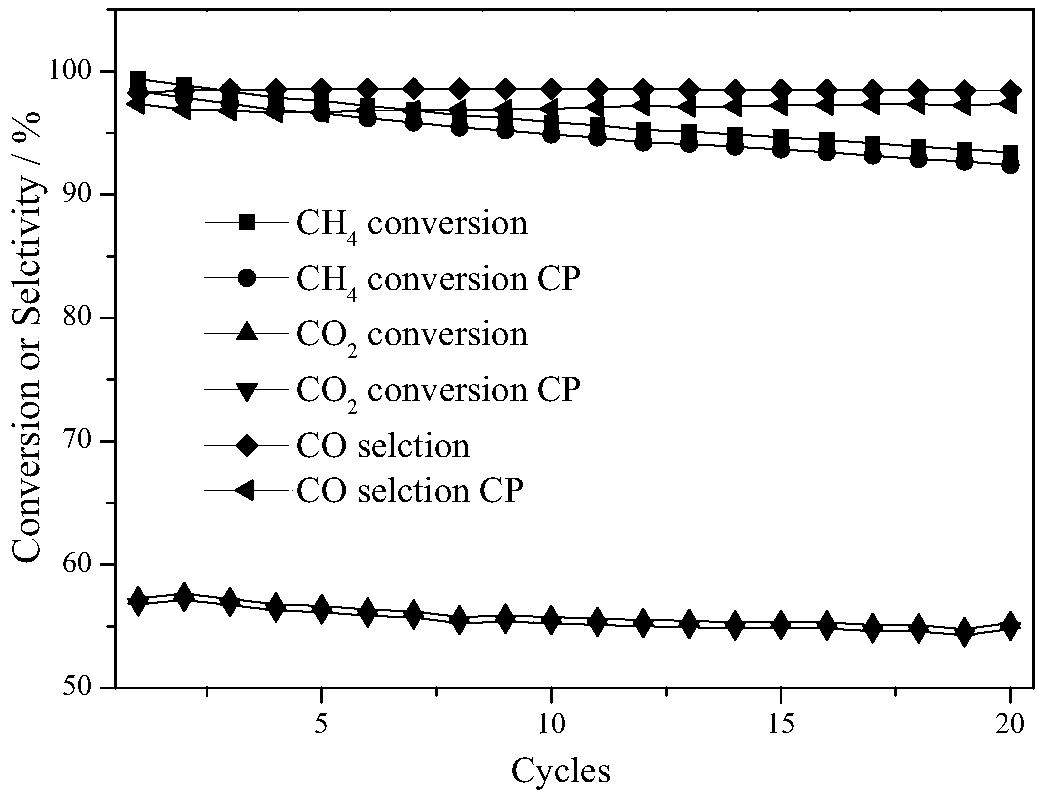
ActiveCN108855109AReduce contentReduce reactivityHeterogenous catalyst chemical elementsHydrogen productionPartial oxidationHydrogen
The invention relates to an oxygen carrier applied to a chemical looping partial oxidation process for preparing synthesis gas by using methane, and a preparation method and application of the oxygencarrier. The preparation method comprises the steps of adding a LaFeO3 carrier into a nickel nitrate solution, soaking, evenly stirring and then drying; after that, calcining for 1-12h at 400-800 DEGC so as to obtain the oxygen carrier. When the oxygen carrier is applied to the chemical looping partial oxidation process for preparing the synthesis gas by using the methane, a fuel reactor and theregeneration reactor which are communicated with each other are adopted, and the oxygen carrier is circulated between the fuel reactor and the regeneration reactor; the oxygen carrier is simple in preparation method and good in repeatability, can utilize the high selectivity (greater than 90%) of self lattice oxygen in a wider temperature to perform partial oxidation on the methane for producing the synthesis gas, and can be regenerated in various oxidizing atmospheres such as air, water vapor, carbon dioxide, water / carbon dioxide gas mixture; meanwhile, high value-added products such as highpurity hydrogen, carbon monoxide or synthesis gas can be produced. After being subjected to a circular reaction for a plurality of times, the oxygen carrier is higher in reaction activity and synthesis gas selectivity.
Owner:NORTHWEST UNIV(CN)
Application of three-dimensional ordered macro-porous perovskite type oxide in preparing hydrogen through carbonic fuel chemical chain
InactiveCN102515096AStable structureIncrease contact areaHydrogenBulk chemical productionWater vaporDecomposition
The invention provides an application of a three-dimensional ordered macro-porous perovskite type oxide which is used as an oxygen carrier for preparing hydrogen through a carbonic fuel chemical chain. The three-dimensional ordered macro-porous perovskite type oxide has the characteristics of the perovskite type oxide and has a three-dimensional ordered macro-porous structure of an inverse opal structure. The three-dimensional ordered macro-porous perovskite type oxide is used as an oxygen carrier for preparing the hydrogen through the carbonic fuel chemical chain and has a general formula of ABO3, wherein A is rare-earth metal and B is transition metal. The three-dimensional ordered macro-porous structured perovskite structure has stable properties and longer service life; pore channels and specific surface area are bigger; during a contact process of the oxygen carrier and a carbonic fuel, much more lattice oxygen for participating in reaction can be supplied; the contact of the oxygen carrier and the carbonic fuel can be boosted; the specific surface area of the oxygen carrier can be increased by abundant pore channels; catalytic conversion between small particles and gas molecules is boosted; the three-dimensional ordered macro-porous perovskite type oxide has higher reaction activity in chemical chain combustion and steam pyrolytic decomposition; and compared with the conventional metal oxygen carrier, the three-dimensional ordered macro-porous perovskite type oxide has higher reaction activity and hydrogen yield.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
Low-carbon alkane chemical chain oxydehydrogenation to olefin technology
InactiveCN108046973ALimit optionsHigh energy consumptionCatalyst regeneration/reactivationHydrocarbonsWater vaporLattice oxygen
The invention relates to a design of low-carbon alkane (C2-C5) chemical chain oxydehydrogenation to olefin technology. A catalyst adopted by the technology is a novel bifunctional catalyst, a metallicoxide with an oxygen-carrying function is used as an oxygen carrier to provide lattice oxygen for oxidative dehydrogenation, and a metal active ingredient with a dehydrogenation function is loaded onthe metallic oxide. According to the technology, a fixed bed, a circulating fluidized bed or a moving bed can be taken as a reactor, in the oxidative dehydrogenation process, the metal active ingredient can be taken as a dehydrogenation active center, the reaction of dehydrogenation of alkane to olefin is taken place in the reactor, the lattice oxygen carried by the oxygen carrier can be selectively reacted with H2 generated in the dehydrogenation to oxidize the H2 to steam, when the reaction is completed, the catalyst losing the lattice oxygen is oxidized and regenerated in the air to supplement the lattice oxygen while carbon deposit is removed, and the reactivity of the catalyst is recovered. Compared with a traditional technology, in situ oxidation is conducted on the H2 generated inthe dehydrogenation to greatly improve percent conversion of the alkane and olefin yield, demands of catalytic reaction and catalyst regeneration can be met simultaneously, the dehydrogenation can beconducted at a lower temperature, the oxidized and regenerated catalyst carries a large amount of heat which can be provided for the dehydrogenation to achieve an operation of self-heating in the dehydrogenation process, and energy consumption can be greatly reduced by means of the designs.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Fluidizable vanadium catalyst for oxidative dehydrogenation of alkanes to olefins in a gas phase oxygen free environment
InactiveUS9878305B2Organic compound preparationHeterogenous catalyst chemical elementsAlkaneLattice oxygen
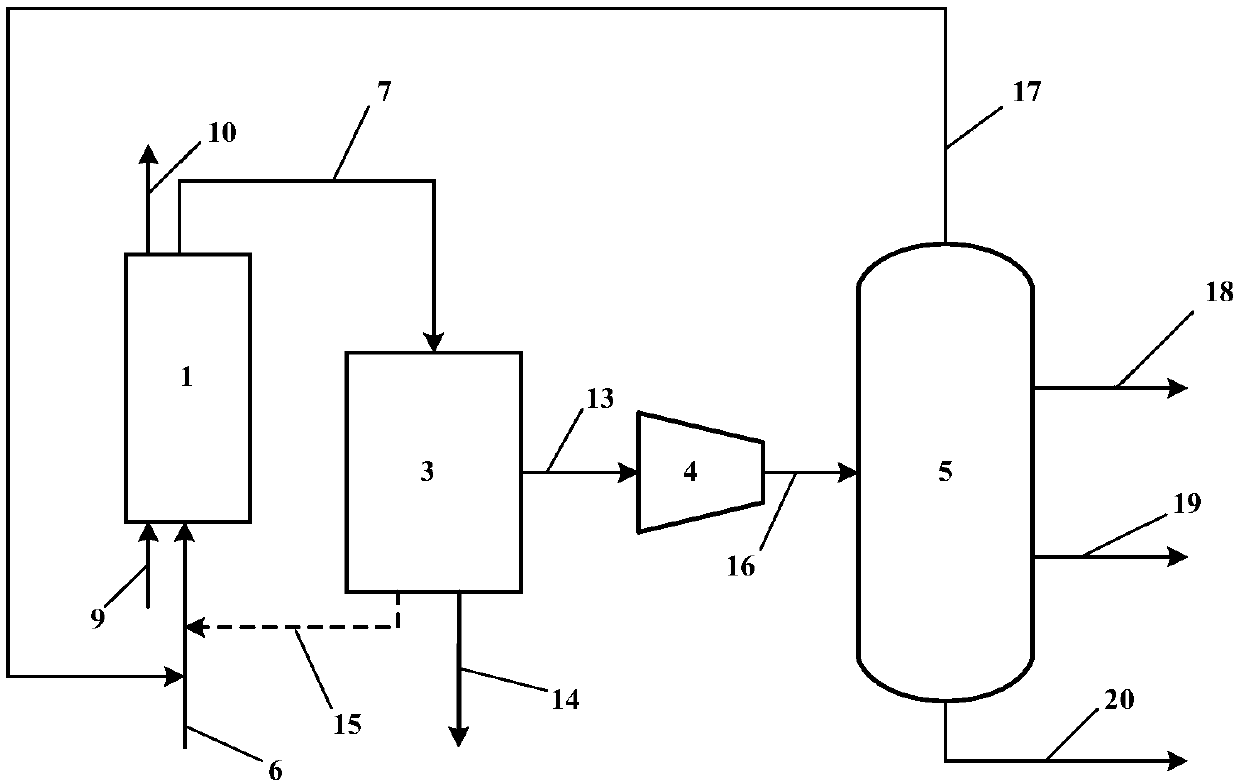
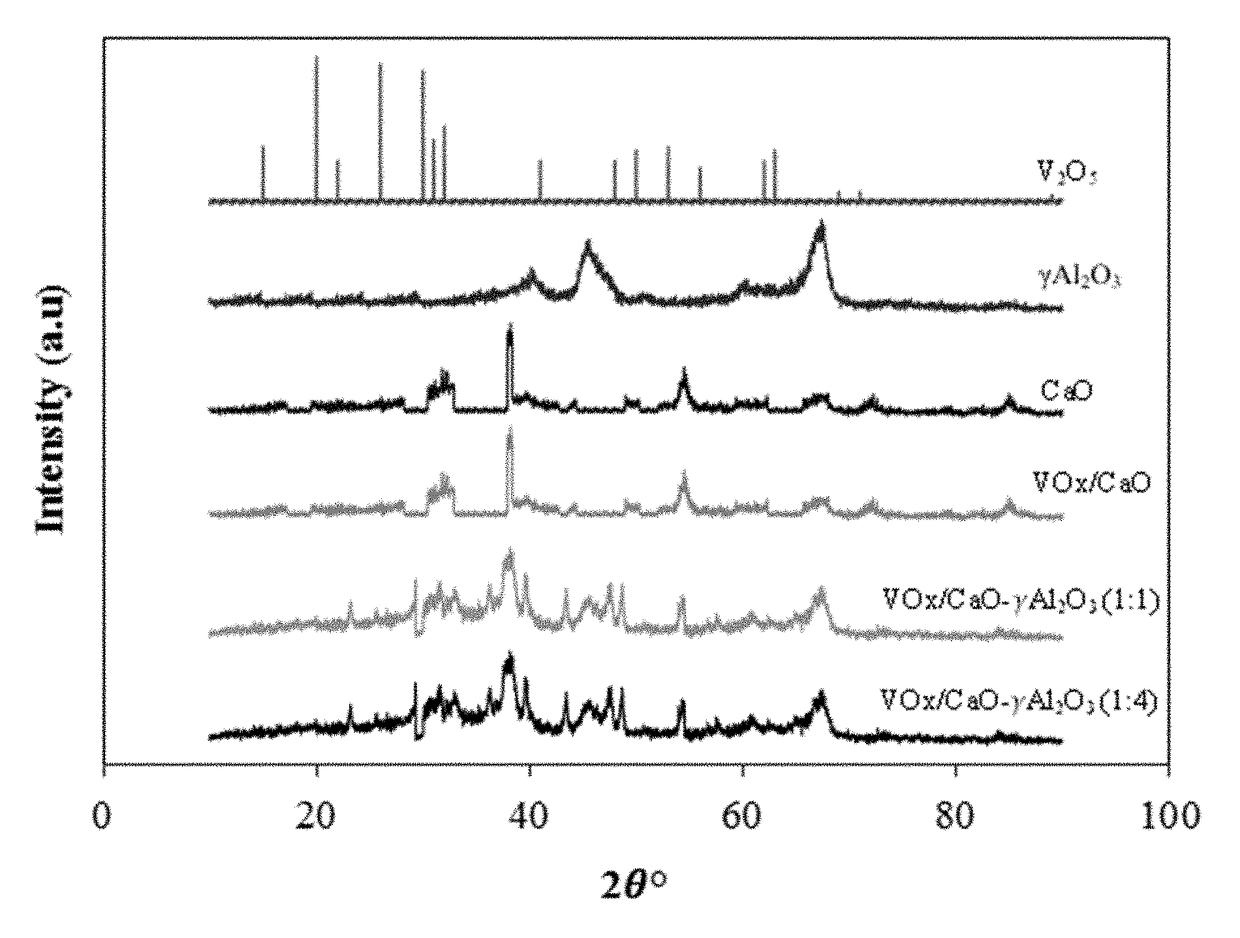
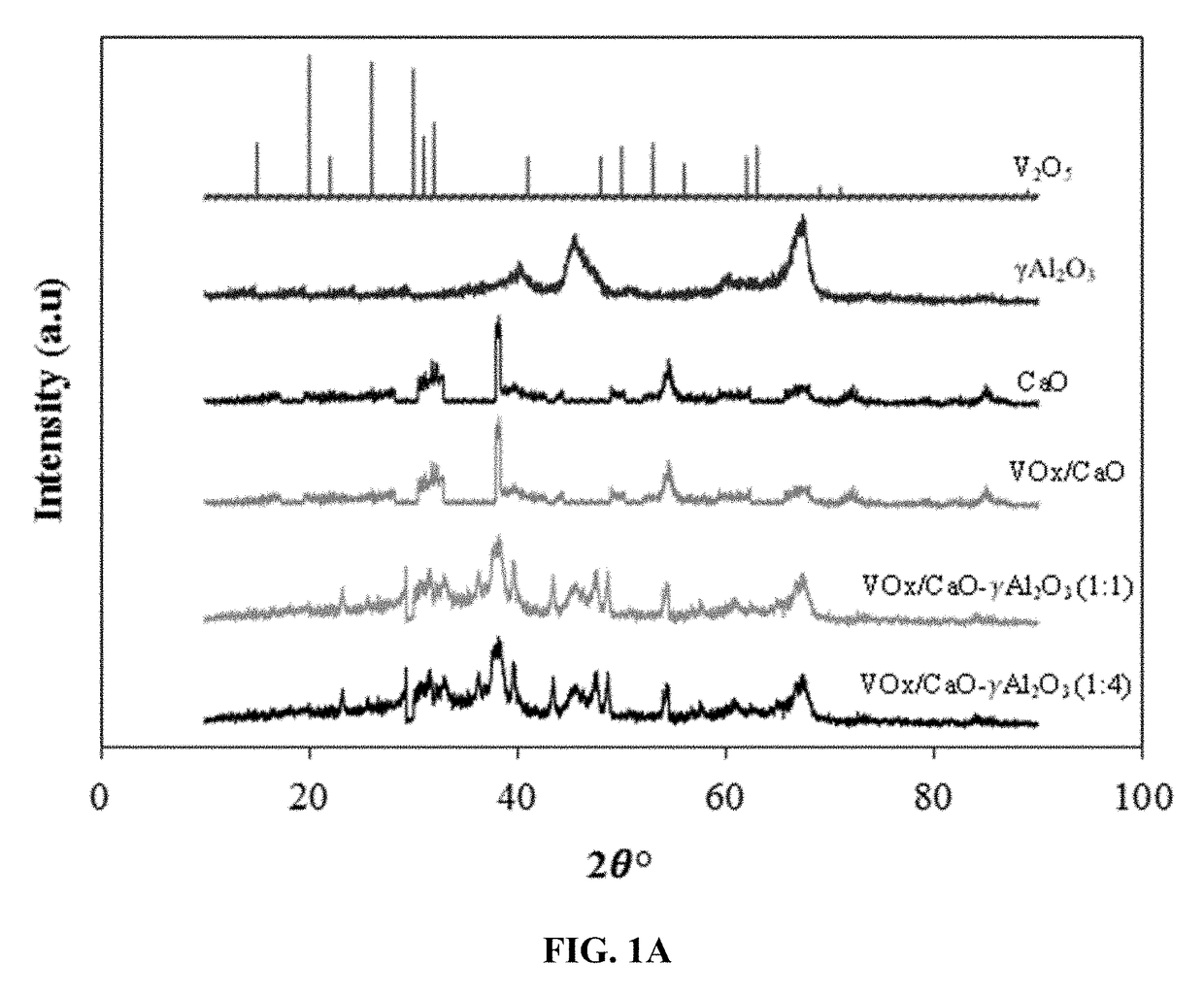
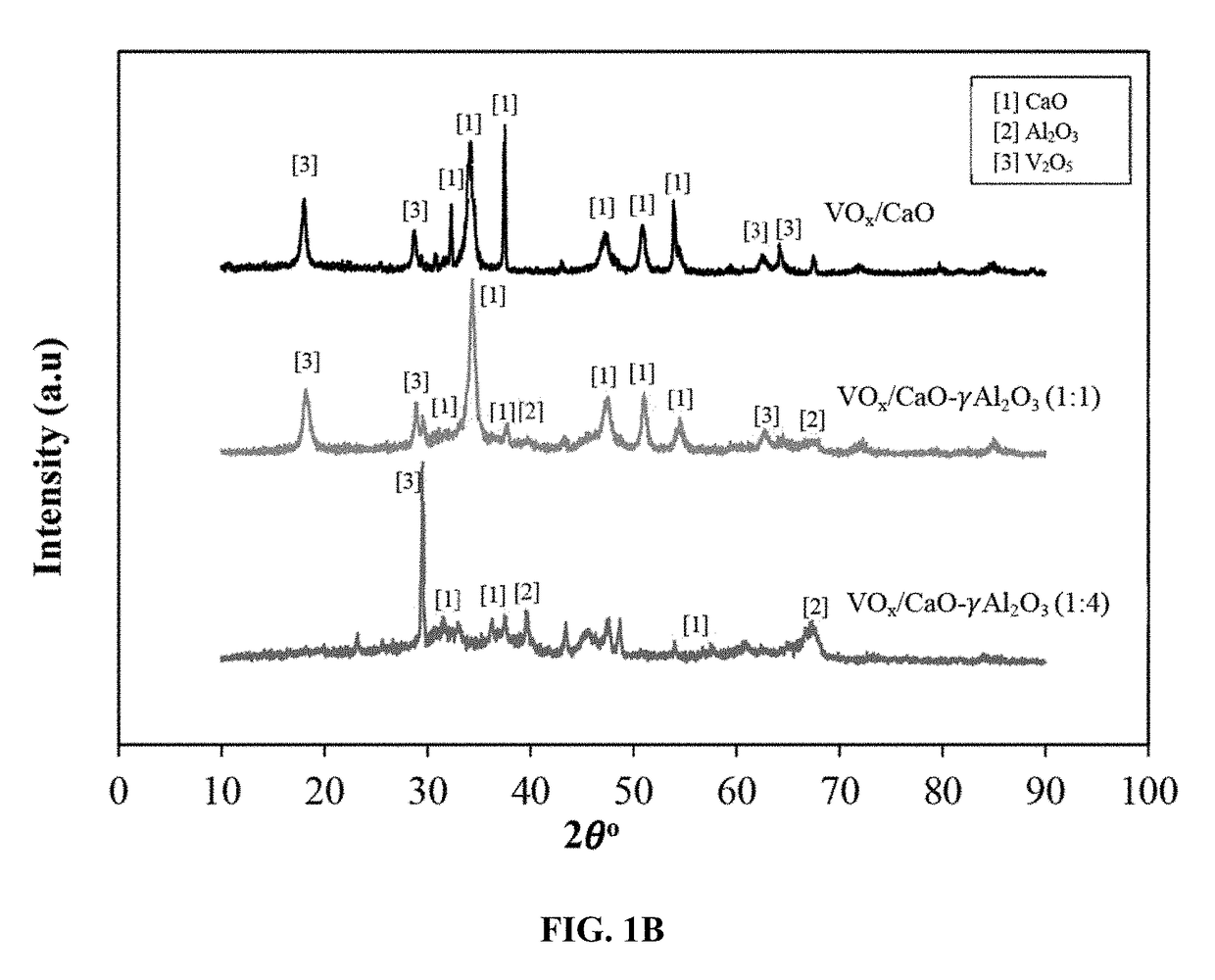
Fluidizable catalysts for the gas phase oxygen-free oxidative dehydrogenation of alkanes, such as propane, to corresponding olefins, such as propylene. The catalysts comprise 5-20% by weight per total catalyst weight of one or more vanadium oxides (VOx), such as V2O5. The dehydrogenation catalysts are disposed on an alumina support that is modified with calcium oxide to influence characteristics of lattice oxygen at the catalyst surface. Various methods of preparing and characterizing the catalyst as well as methods for the gas phase oxygen free oxidative dehydrogenation of alkanes, such as propane, to corresponding olefins, such as propylene, with improved alkane conversion and olefin product selectivity are also disclosed.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
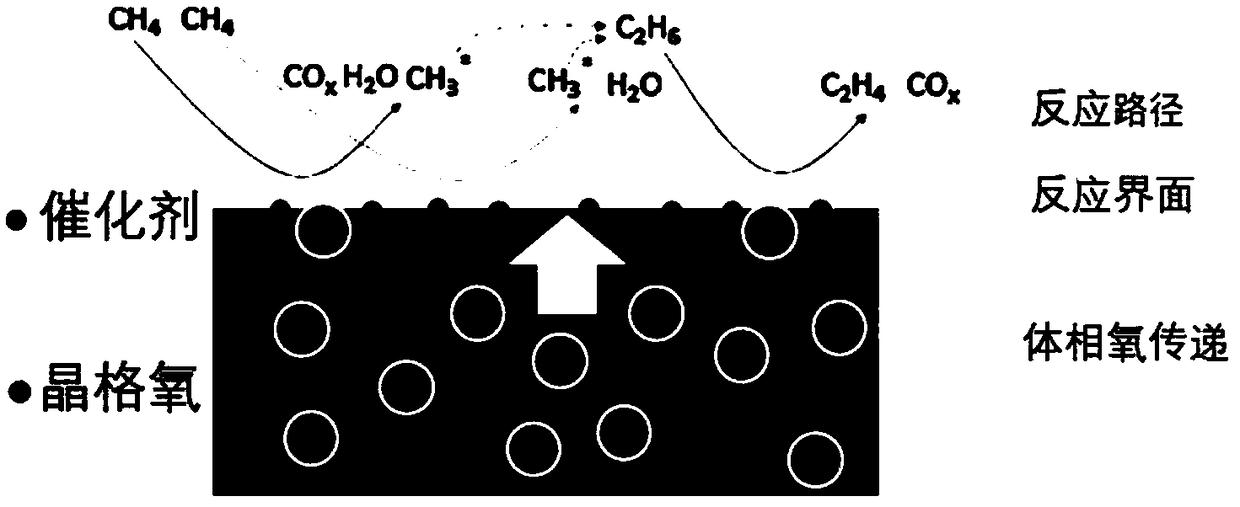
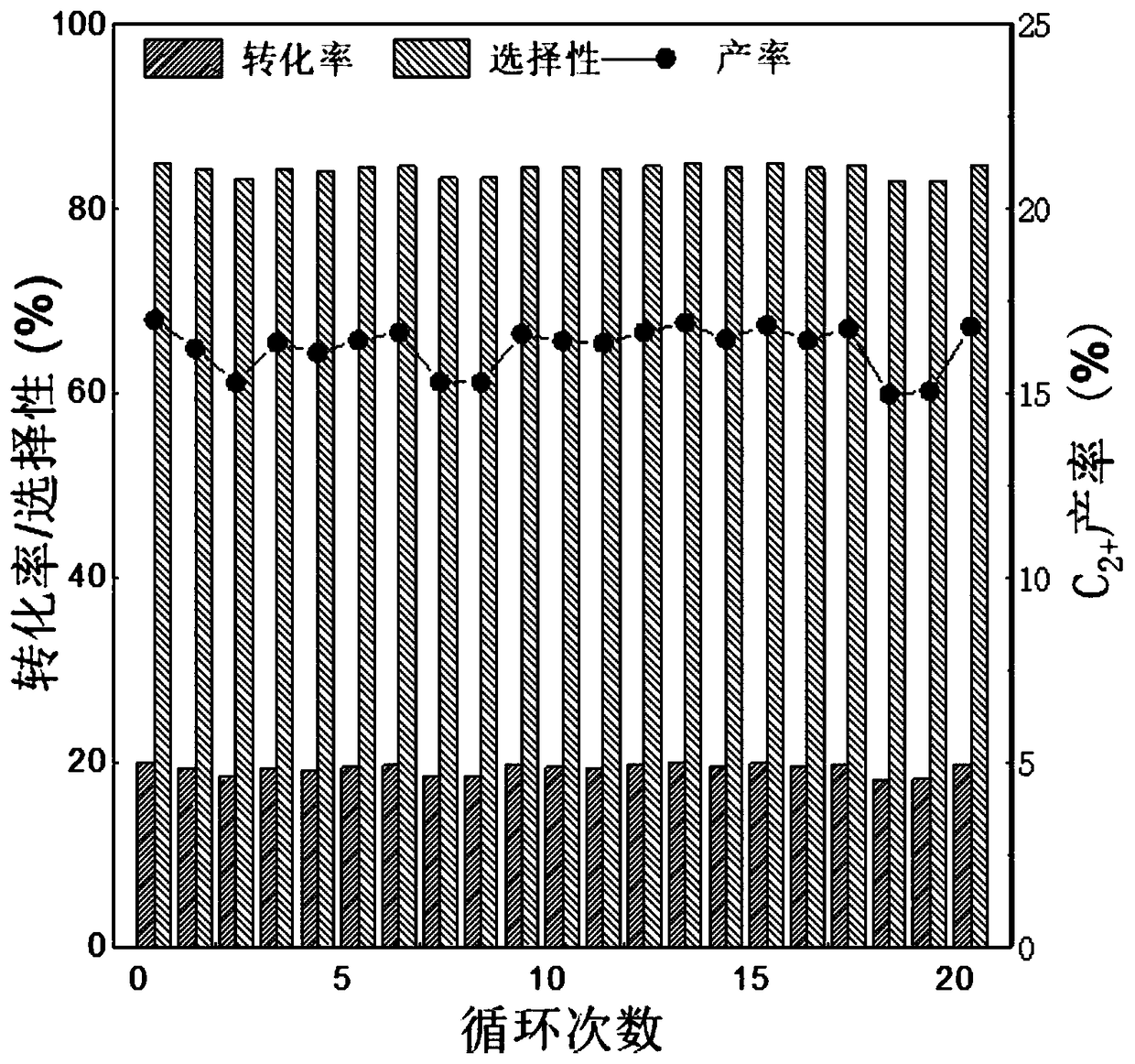
Methane oxidative coupling method based on chemical chain lattice oxygen transfer technology
ActiveCN109438159AReduce over-oxidationAvoid direct contactCatalystsHydrocarbon preparation catalystsLattice oxygenCoupling
The invention belongs to the technical field of methane conversion, and particularly relates to a methane oxidative coupling method based on the chemical chain lattice oxygen transfer technology. According to the method, one-step high-efficiency methane oxidative coupling is realized through utilization of a composite material with the functions of catalytically cracking methane and selectively supplying oxygen; catalytic oxygen supply materials include methane cracking catalysts and selective oxygen supply materials, the catalysts provide active sites for activated cracking of the methane, the oxygen supply materials partially supply oxygen for reaction by transferring oxygen atoms through lattice oxygen, and catalytic oxygen carriers after the reaction are regenerated by free oxygen oxidation; oxygen transfer in the reaction is realized through circulation of redox reaction of the catalytic oxygen carriers, and direct contact between free oxygen and the methane is avoided; the lattice oxygen activity is controlled directionally, excessive oxidation of the methane is weakened, and selectivity and yield of olefin are increased; the methane oxidative coupling has high reactivity andselectivity at the temperature of 800-850 DEG C.
Owner:SOUTHEAST UNIV
Alkane crystal lattice oxygen selectivity oxidized activating catalyze cracking catalyst and method of use thereof
InactiveCN101249455AReduce energy consumptionReduce dosageCatalytic crackingMolecular sieve catalystsAlkaneWater vapor
The invention relates to an alkane catalytic cracking catalyst of lattice oxygen selective oxidation and activation and application method thereof. The method mainly includes oxidizing and activating alkane in naphtha with the lattice oxygen in the catalyst to produce oxygen-containing compound which is easy to be cracked, and carrying out catalytic cracking on cracking active sites to produce ethylene and propylene at high conversion rate and high selectivity. Compared with the prior art, the inventive catalyst has greatly reduced reaction temperature and greatly reduced water vapor amount (5wt% of naphtha), thereby greatly reducing the energy consumption of reaction part; high propylene yield and low ethylene yield, thereby improving the ratio of propylene to ethylene; less resultant methane with low added value and high yield of high added value product, thereby facilitating the efficient utilization of raw material; and less resultant methane, ethane and ethylene to greatly reduce material requiring cryogenic separation, thereby obviously reducing energy consumption of compression and separation system.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Preprocessed optical coating materials and preprocessed method thereof
The invention discloses a pretreated optical coating material and its pretreated methods. The methods includes: (1) selecting the optical coating powder, (2) preparing simple blank at a temperature of 700-1400DEG C, pressure of 10-40Mpa, (3) sintering in a high-temperature sintering furnace at 900-1800DEG C for 2-4h for the preparation bodyware; (4)processing and forming the bodyware strictly in accordance with coater crucible size, (5) degassing in the reduction furnace, at a temperature of 1000-1700DEG C, for 2-6hours to obtain the pretreated optical coating materials. The TiO2 pretreated optical coating materials has a relative density of 99%, oxygen content of 39.8%, oxygen loss rate of 6%, by fitting its stoichiometric formula, its molecular formula is TiO1.979. Pretreated optical coating material has the following characteristics: 1) high pyknosis with relative density over 90%, close to the theoretical density; 2) losing some lattice oxygen according to high refractive index optical oxide coating materials.
Owner:有研资源环境技术研究院(北京)有限公司
High-performance oxygen adsorbent and preparation method thereof
InactiveCN104857911AOther chemical processesDispersed particle separationElectrical conductorDesorption
The invention relates to a high-performance oxygen adsorbent and a preparation method thereof, wherein the oxygen adsorbent material is a mixed conductor ceramic material having oxygen selectivity, and provides extremely high oxygen selectivity by using lattice oxygen vacancy to carry out chemical adsorption and desorption on oxygen when the temperature is increased or decreased. The production method comprises: uniformly mixing raw materials according to a ratio, calcining for a certain time at a high temperature to prepare large-particle oxide powder, and carrying out double ball milling to refine the particles so as to prepare the multi-metal composite oxide powder. The novel oxygen adsorbent of the present invention can be used for temperature changing oxygen adsorbing and has high oxygen adsorption.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Ce/Zr composite oxide, and preparation method and application thereof
ActiveCN109529802AEvenly distributedLarge specific surface areaDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsRare-earth elementComposite oxide
The invention relates to a Ce / Zr composite oxide, and a preparation method and an application thereof. The Ce / Zr composite oxide includes, by weight, 30-70% of cerium oxide, 20-60% of zirconium oxide,5-50% of a metal oxide having L acidity, 3-5% of oxides of other rare earth elements, and 3-10% of sulfate ion. The preparation method includes: processing corresponding source materials of the Ce / Zrcomposite oxide through a sol method to prepare a sol liquid and performing hydrothermal treatment. The Ce / Zr composite oxide simultaneously has B-L dual-acidity, so that the composite has very highpurification effect on nitrogen-containing compounds and unsaturated hydrocarbon compounds. Meanwhile, the composite oxide has uniform distribution of metal elements, is high in specific surface area,has good anti-aging effect and is long in service life. By introducing the metal oxide having L acidity, large lattice imperfection can be formed, and oxygen storage capacity of the lattices is increased, so that the composite oxide is improved in adsorption capacity on sulfides, so that poisoning of the noble metals is prevented and service life of a composite catalyst is prolonged.
Owner:SHANDONG SINOCERA FUNCTIONAL MATERIAL CO LTD
Molybdenum-vanadium double-metal oxide catalyst and application of same to chemical-chain dehydrogenation of light alkanes
ActiveCN109382090AHigh selectivityImprove conversion rateCatalyst regeneration/reactivationCatalystsAlkaneLattice oxygen
The invention discloses a molybdenum-vanadium double-metal oxide catalyst and application of the same to chemical-chain dehydrogenation of light alkanes. The molybdenum-vanadium double-metal oxide catalyst has a molecular formula of Mo1Vy, wherein y represents a molar ratio of vanadium atoms to molybdenum atoms; the molybdenum-vanadium double-metal oxide catalyst is prepared by using a dipping method; and the Mo1Vy oxygen carrier is prepared through dipping, drying, calcining and tableting. When the loaded molybdenum-vanadium double-metal oxide is applied to the dehydrogenation reaction of light alkanes for preparation of olefins and a reaction temperature is kept at 450-550 DEG C, high-activity high-selectivity oxidative dehydrogenation of propane into propylene can be achieved, the conversion rate of propane is maintained at 30 -40%, and the selectivity of propylene is 80-90%. After the fresh oxygen carrier reacts with propane, the fresh oxygen carrier turns from a high valence stateto a low valence state; a low-valence-state oxygen carrier reacts with air or oxygen and is oxidized into a high valence state, and lattice oxygen is regained and is circulated again; and after repeated regeneration, the oxygen carrier still has stable reaction performance. The catalyst provided by the invention can be used in reaction units such as fixed-bed reactors, moving-bed reactors or circulating fluidized beds.
Owner:TIANJIN UNIV
Preparation method for fluorine-doping Li4Ti5O12 nanosheet
ActiveCN105845924AIncrease contact areaImprove conductivityMaterial nanotechnologyAlkali titanatesHigh rateLattice oxygen
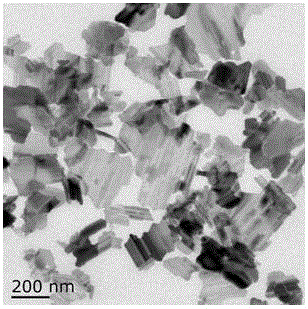
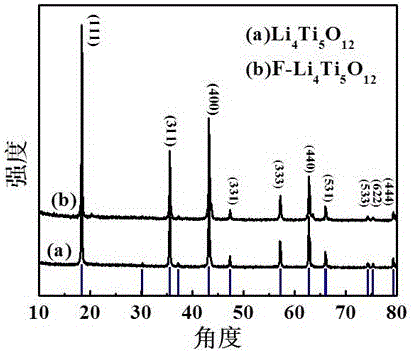
The invention relates to a preparation method for a fluorine-doping Li4Ti5O12 nanosheet, and belongs to the technical field of production of an energy material of a lithium ion battery. The Li4Ti5O12 nanosheet is synthesized by a hydrothermal method, thus, the contact surface between a material and an electrode can be remarkably expanded, and the electrochemical performance of the material can be further improved; with the doping of fluorine atoms, a part of lattice oxygen atoms can be substituted by the fluorine atoms to form Ti-F bonds; according to charge conservation, the material charge is out of balance due to the introduction of F; in order to ensure the charge conservation of the material, a part of Ti<4+> can be converted to Ti<3+>, so that the conductivity of the material is improved; and through the doping of F, the obtained product is uniform in morphology, high in crystallinity and large in specific area, and the electrochemical performance at high rate can be remarkably improved.
Owner:YANGZHOU UNIV
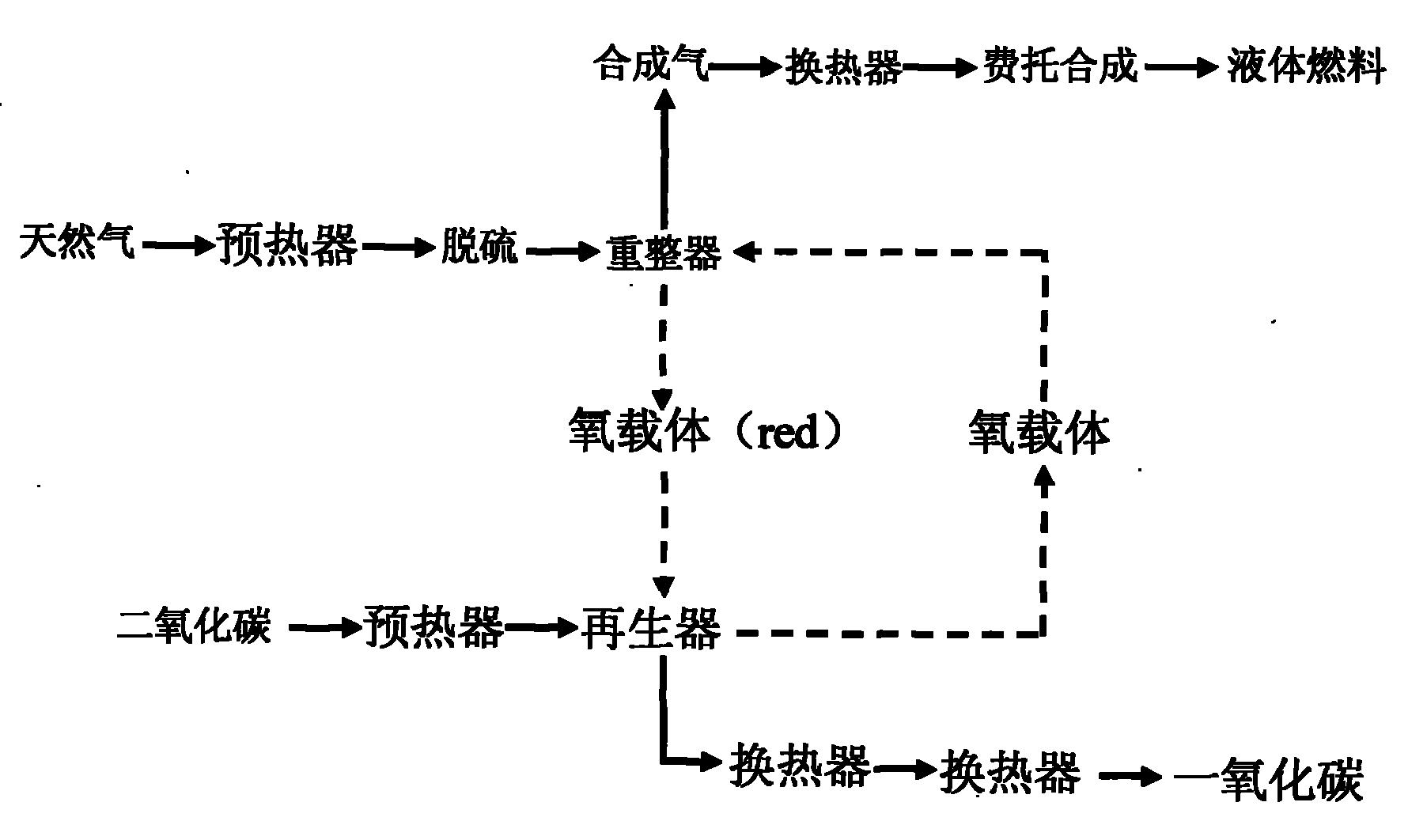
Method for producing synthetic gas by natural gas conversion
InactiveCN101830434AEfficient conversionContinuous conversionHydrogenThermochemical cycleLattice oxygen
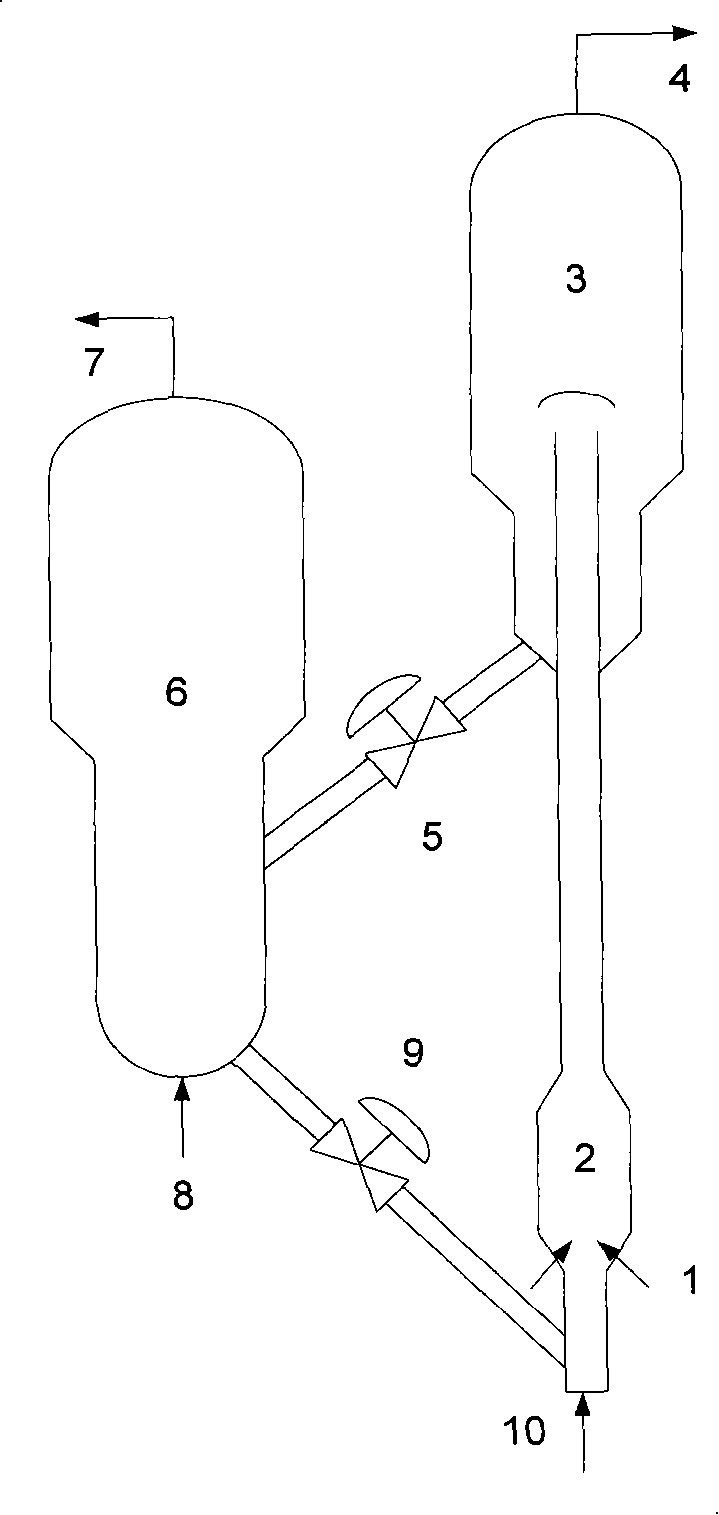
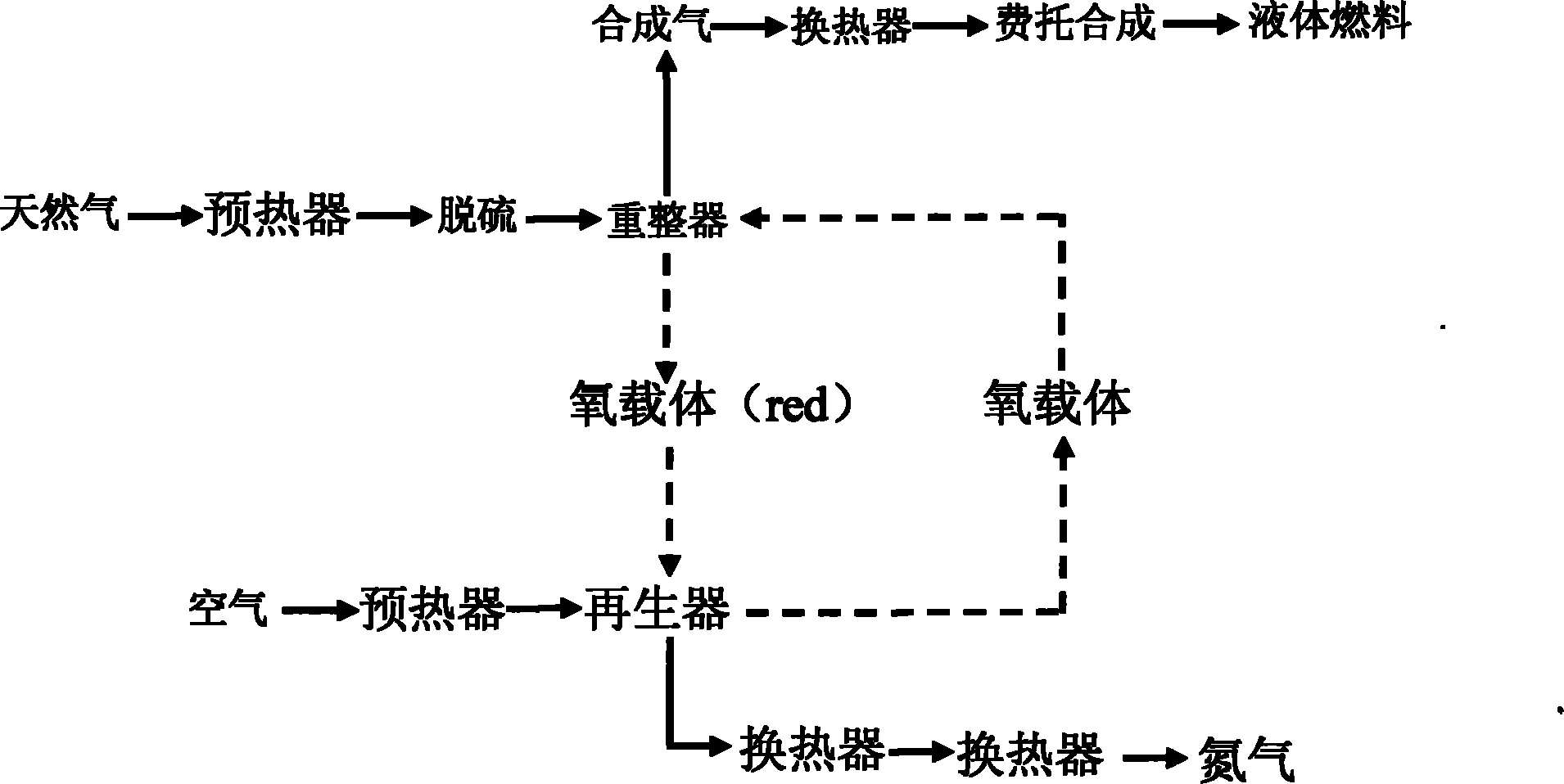
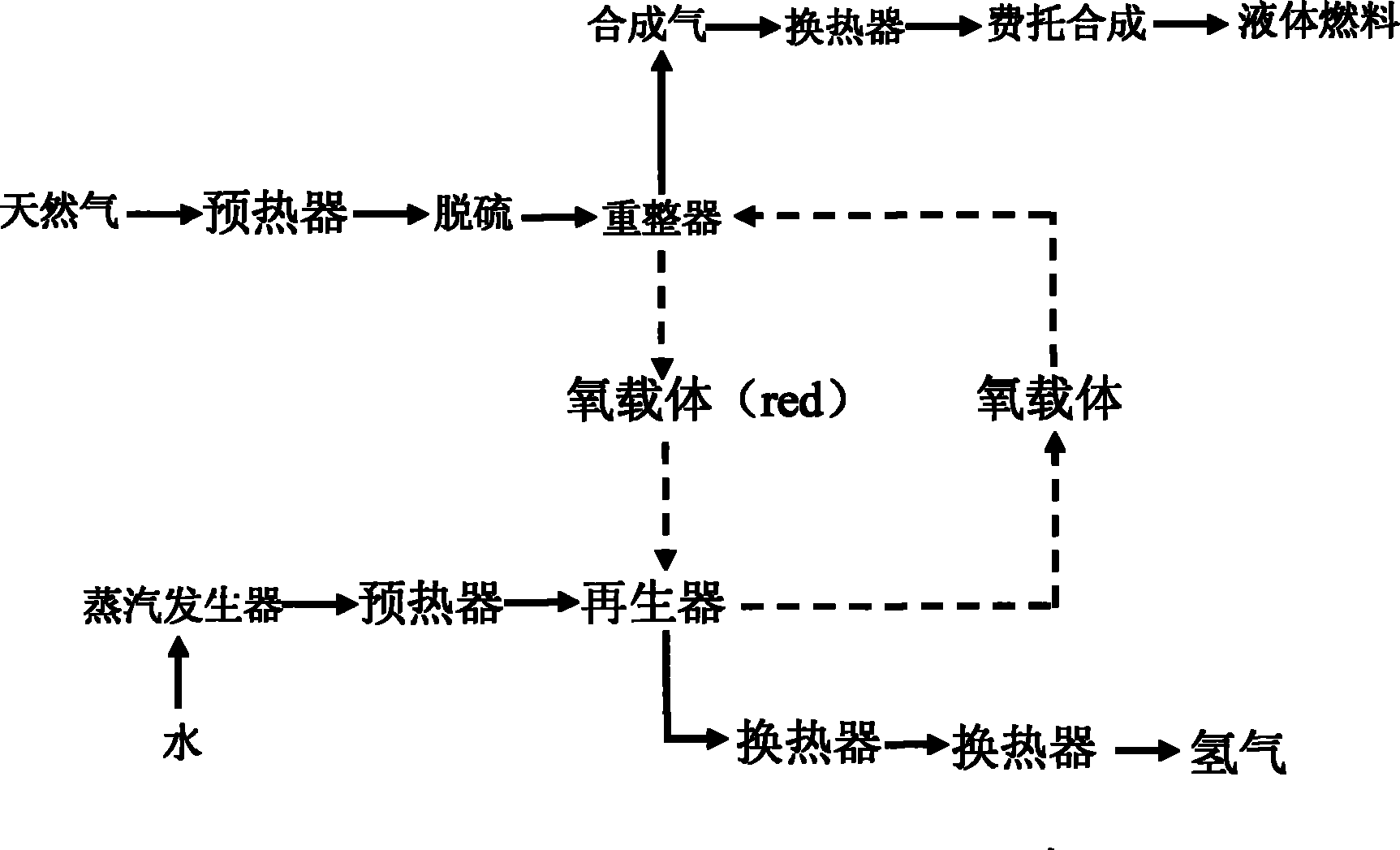
The invention discloses a circulating fluidized bed technology-based oxygen carrier thermo-chemical circulation process aiming at the purpose of producing synthetic gas by natural gas scale conversion. Natural gas is introduced at the inlet of a reforming reactor of a circulating fluidized bed at a certain speed, the natural gas captures lattice oxygen of oxygen carriers in the reforming reactor, and the generated synthetic gas (H2 and CO) can be used for preparing liquid fuel by Fischer-Tropsch synthesis after heat exchange of a heat exchanger and purification; and meanwhile, the oxygen carriers losing the lattice oxygen rise along with airflow and drop and return to an oxygen carrier regenerating reactor to react with an oxygen source such as H2O, air or CO2 and the like introduced from the bottom so as to recover the lattice oxygen thereof, the generated H2, N2 and CO airflow take the regenerated oxygen carriers out of the regenerating reactor and drop the oxygen carriers to the reforming reactor, and the H2, N2 and CO are discharged from the top and used as products through heat exchange of the heat exchanger and purification. The oxygen carriers are circularly used in two reaction areas of the circulating fluidized bed. The technology provides an important path for producing the synthetic gas by the natural gas scale conversion.
Owner:KUNMING UNIV OF SCI & TECH
Nanorod-like low-temperature denitration catalyst and preparation method thereof
ActiveCN105618031AHigh activityLarge specific surface areaMaterial nanotechnologyHeterogenous catalyst chemical elementsMANGANESE ACETATEWater baths
The invention discloses a nanorod-like low-temperature denitration catalyst. TiO2 of anatase is taken as a carrier and manganite is taken as an active component; the length-diameter ratio of nanorod is (10 to 20):1. The nanorod-like low-temperature denitration catalyst is prepared by adopting an improved sol-gel method and is particularly prepared by the following steps of firstly, uniformly mixing and stirring tetra-n-butyl titanate, ethanol, acetic acid and ethyl acetoacetate, adding a template agent for fully stirring, dropping a manganese acetate solution into a mixed solution, and dropping while stirring; after the dropping is finished, continuously stirring, and then heating an obtained solution in a water bath to obtain a gel substance; drying the gel substance, performing roasting treatment on the dried gel substance, and finally performing ultraviolet radiation on a product to obtain a final product. Compared with a traditional MnOx / TiO2 low-temperature denitration catalyst, the nanorod-like low-temperature denitration catalyst prepared by a preparation method disclosed by the invention has the characteristics of better nanorod-like structure, greater specific surface area, more Lewis acid sites, higher lattice oxygen content, high removal rate of nitrogen oxide and the like.
Owner:WUHAN UNIV OF TECH
Catalyst for chemical looping partial oxidation of methane to syngas, preparation and application thereof
ActiveCN109833877AHave substantive characteristicsHigh reactivityHydrogenChemical recyclingPartial oxidationWater vapor
The invention relates to a catalyst for chemical looping partial oxidation of methane to syngas, in particular to a composite oxide containing lanthanum, strontium, iron and aluminum, preparation andapplication thereof. Different metal precursors are mixed evenly in certain proportion and then roasted at certain temperature for certain period of time, thus obtaining the composite oxide catalyst.The catalyst provided by the invention has the characteristics of simple preparation method and good repeatability, can achieve high selectivity (greater than 90%) oxidation of methane to syngas in awide temperature range with the lattice oxygen of itself, also can be regenerated in air, water vapor, carbon dioxide, water / carbon dioxide mixed gas and other oxidizing atmosphere, and at the same time high-purity hydrogen, carbon monoxide or syngas and other high value-added products can be generated. After repeated circulation reaction, the catalyst reaction activity and syngas selectivity haveno obvious change, thus showing excellent cyclic stability. The invention provides the high efficiency and low cost catalyst for chemical looping partial oxidation of methane to syngas.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
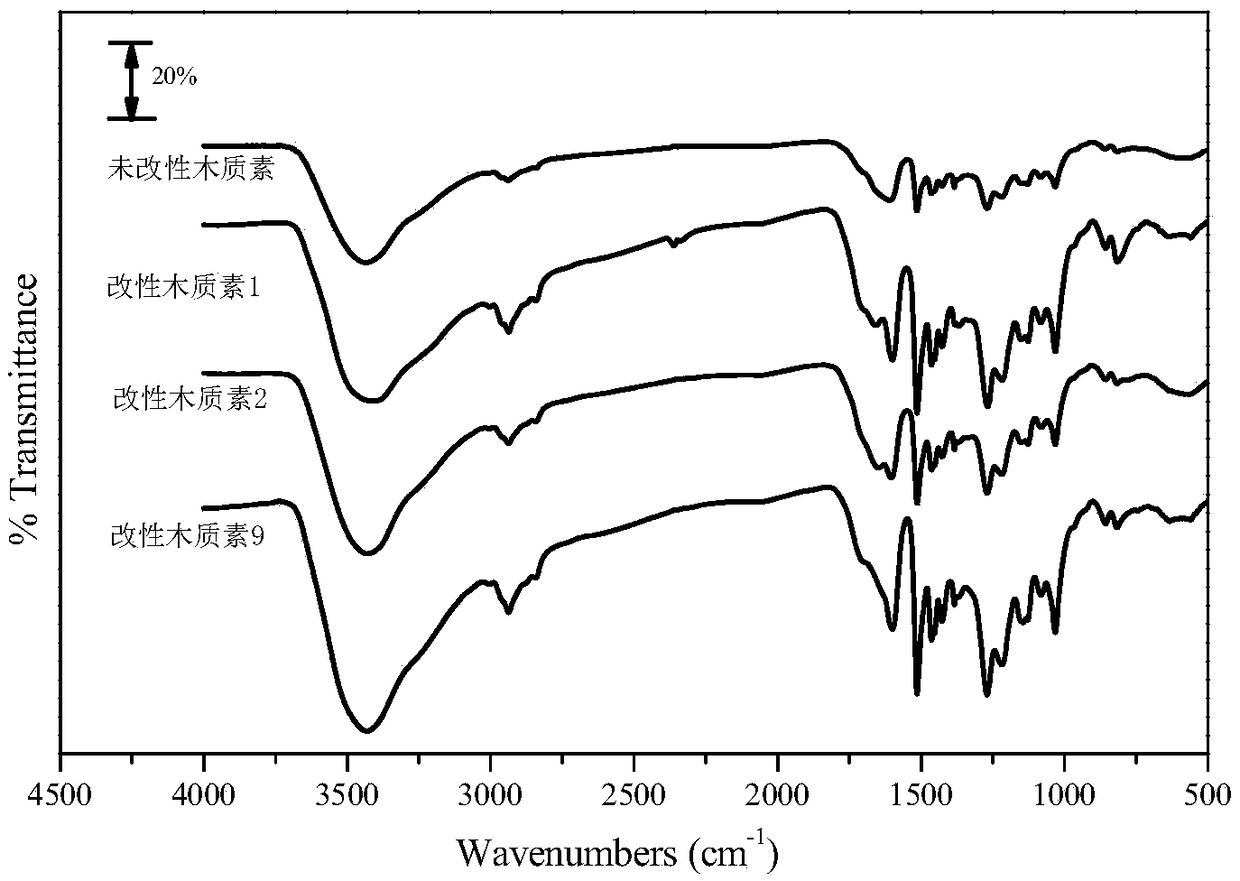
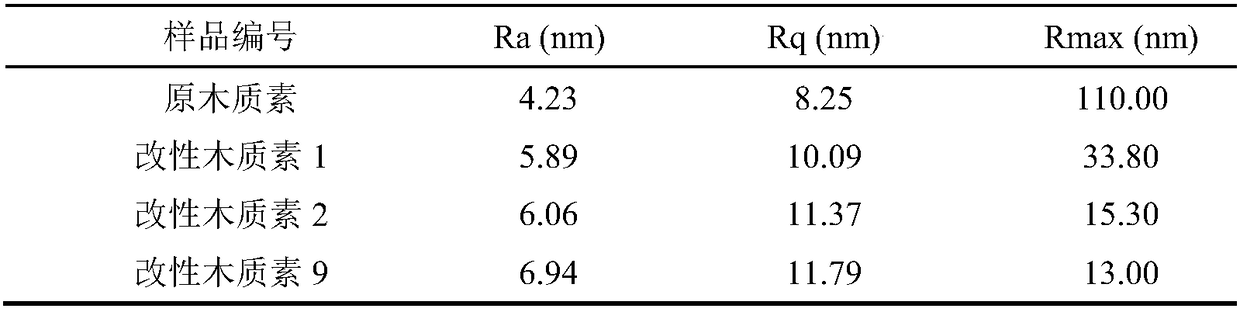
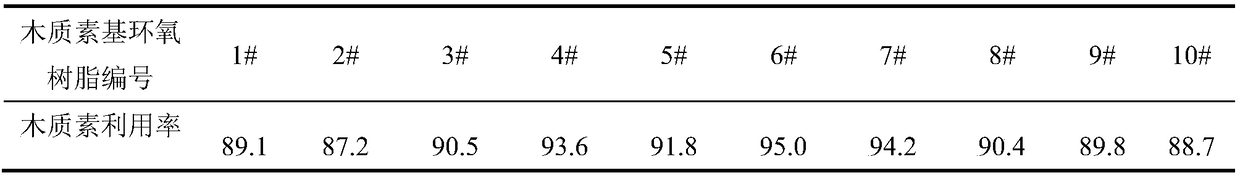
Lignin modification method and preparation method of lignin-based epoxy resin
The invention discloses a lignin modification method, which comprises the following steps: adding lignin and a metal oxide into a eutectoid for modification reaction, and separating the mixture afterthe reaction is finished, so as to obtain modified lignin. The invention further provides a preparation method of lignin-based epoxy resin based on the lignin modification method. According to the lignin modification method disclosed by the invention, the eutectoid is used as a solvent and cooperates with the metal oxide to modify the lignin; a hydrogen bond is formed by using urea and choline chloride in the eutectoid to controllably break C-C and C-O bonds and surface functional groups in a lignin structure to achieve an effect of fully dissolving the lignin, and then lattice oxygen formed in an M=O bond in the metal oxide is used to selectively oxidize the lignin, thereby efficiently catalyzing to remove a methoxy group on the surface of the lignin and the C-O bond in the broken lignin,which achieves the aims of reducing the spatial structural steric hindrance and increasing the surface hydroxyl content and can improve the reactivity of the lignin and epichlorohydrin.
Owner:ANHUI UNIV OF SCI & TECH
Perovskite composite oxide catalyst for preparing methyl alcohol from methane as well as preparation method of catalyst
InactiveCN103949263AEasy to operateShort timePreparation by oxidation reactionsMetal/metal-oxides/metal-hydroxide catalystsComposite oxideOxide
The invention relates to a perovskite composite oxide catalyst for preparing methyl alcohol from methane as well as a preparation method of the catalyst, wherein the perovskite composite oxide catalyst for preparing the methyl alcohol from the methane is a particulate matter, and has the general formula of Me(x)O(y)-A(l-m)A'(m)B(1-n)B'(n)O(3), wherein m is more than or equal to 0 and less than or equal to 1, n is more than or equal to 0 and less than or equal to 1, A is one of Ca and La, A' is one of Ba and Sr, B is one of Fe, Co, Ni and Mn, B' is one of Zr, Ti and Cr, and transition metal oxide Me(x)O(y) is one or two of Fe(2)O(3), N(i)O and Fe(3)O(4). The method provided by the invention is simple to operate, short in consumed time, and lower in operation expense by being compared with the operation expense in a traditional method for preparing the methyl alcohol from the methane. The catalyst provided by the invention can be used for oxidizing crystal lattices to prepare the methyl alcohol by CH4 at 500-500 DEG C; the methane conversion rate is 40-80%, and the methyl alcohol yield rate is 20-40%, and a novel method for preparing the methyl alcohol from the methane is provided.
Owner:NORTHEAST GASOLINEEUM UNIV
Iron-molybdenum composite metal oxide catalyst and preparation method and application thereof
InactiveCN110115995AAvoid direct contactAchieve oxidation regenerationCatalystsHydrocarbon preparation catalystsLattice oxygenReaction temperature
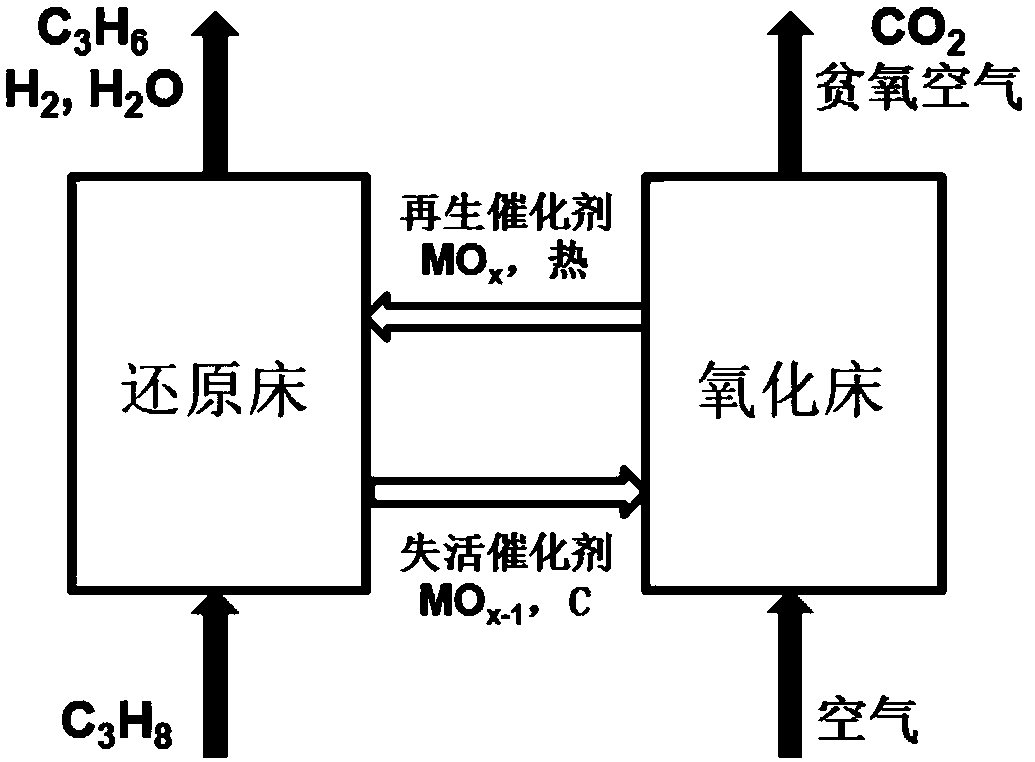
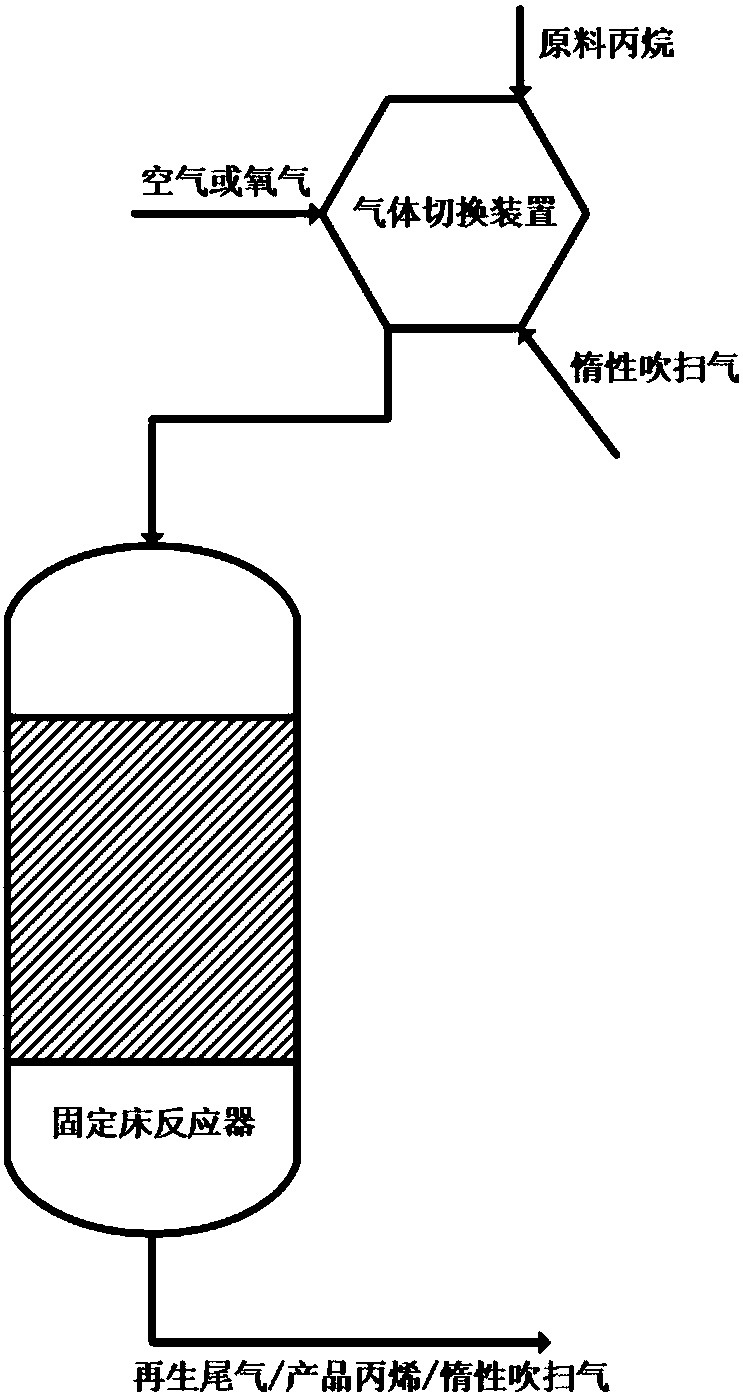
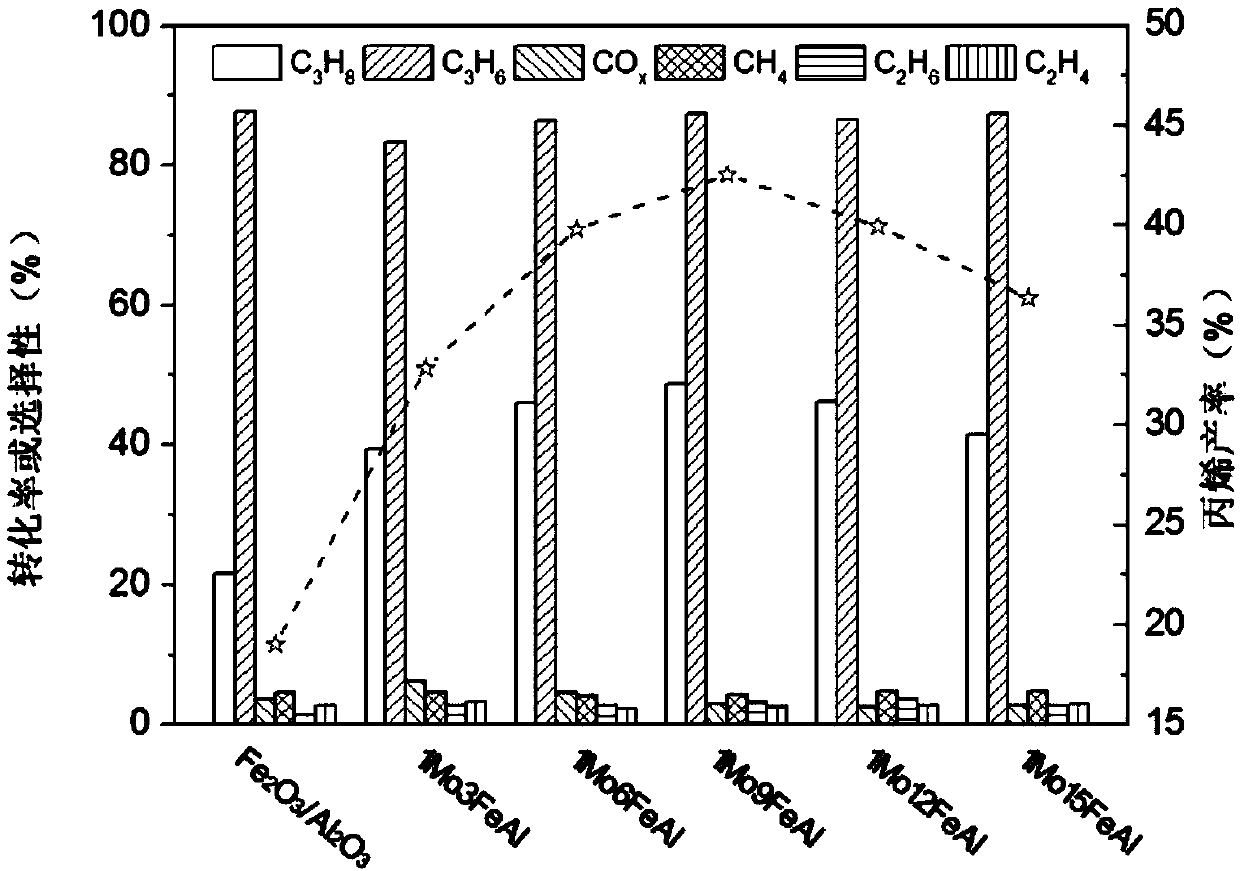
The invention discloses an iron-molybdenum composite metal oxide catalyst and a preparation method and application thereof. Precursors of iron oxide and molybdenum oxide of different amounts are impregnated on an alumina carrier together, and a 1MoxFeAl catalyst is obtained after drying, roasting, tableting and screening, wherein the supporting amount of iron is 3-40%, x represents the molar ratioof iron and molybdenum, and the molar ratio range is 3-15. The catalyst can simultaneously achieve the high conversion rate of propane and the high selectivity of propylene, meanwhile the introduction of lattice oxygen in the catalyst breaks thermodynamic equilibrium and improves the efficiency of the catalyst. After the propane passes through a catalyst bed layer, metal oxide in a high valence state is reduced, the lattice oxygen is lost, the state becomes a low valence state, and catalytic activity is lost. After N2 purification, the reacted catalyst is re-oxidized to be in a high-valence state by introducing air at the reaction temperature, lattice oxygen is supplemented, and g the capacity of oxidative dehydrogenation of propane is regained.
Owner:TIANJIN UNIV
Lithium manganate composite manganous-manganic oxide and industrial preparation method thereof
ActiveCN108059190AAvoid Lattice Oxygen DefectsImprove performanceCell electrodesSecondary cellsLattice oxygenManganous-manganic oxide
The invention discloses lithium manganate composite manganous-manganic oxide and an industrial preparation method thereof. The method comprises the following steps: crushing high-purity manganese metal powder till the particle size of 10-300mu m by using a dry method, grading the manganese metal powder by using grading equipment, putting into a reactor with pure water and an ammonium salt, introducing air for oxidation, stirring at a speed of 100-500r / minute, introducing air at a flow of 100-300m<3> / hour, reacting for 20-25 hours, adding dissoluble manganese salt, sufficiently stirring, further adding bicarbonate, continuously reacting for 3-5 hours till the reaction is completed, further washing, drying, and roasting, thereby obtaining a composite manganous-manganic oxide product. The composite manganous-manganic oxide prepared by using the method is prevented from lithium manganate lattice oxygen defects, product properties and consistence are improved, meanwhile the amount of blownair is reduced, and the energy consumption of a kiln is reduced.
Owner:SINOSTEEL ANHUI TIANYUAN TECH
Thermal energy storage and power generation systems and methods
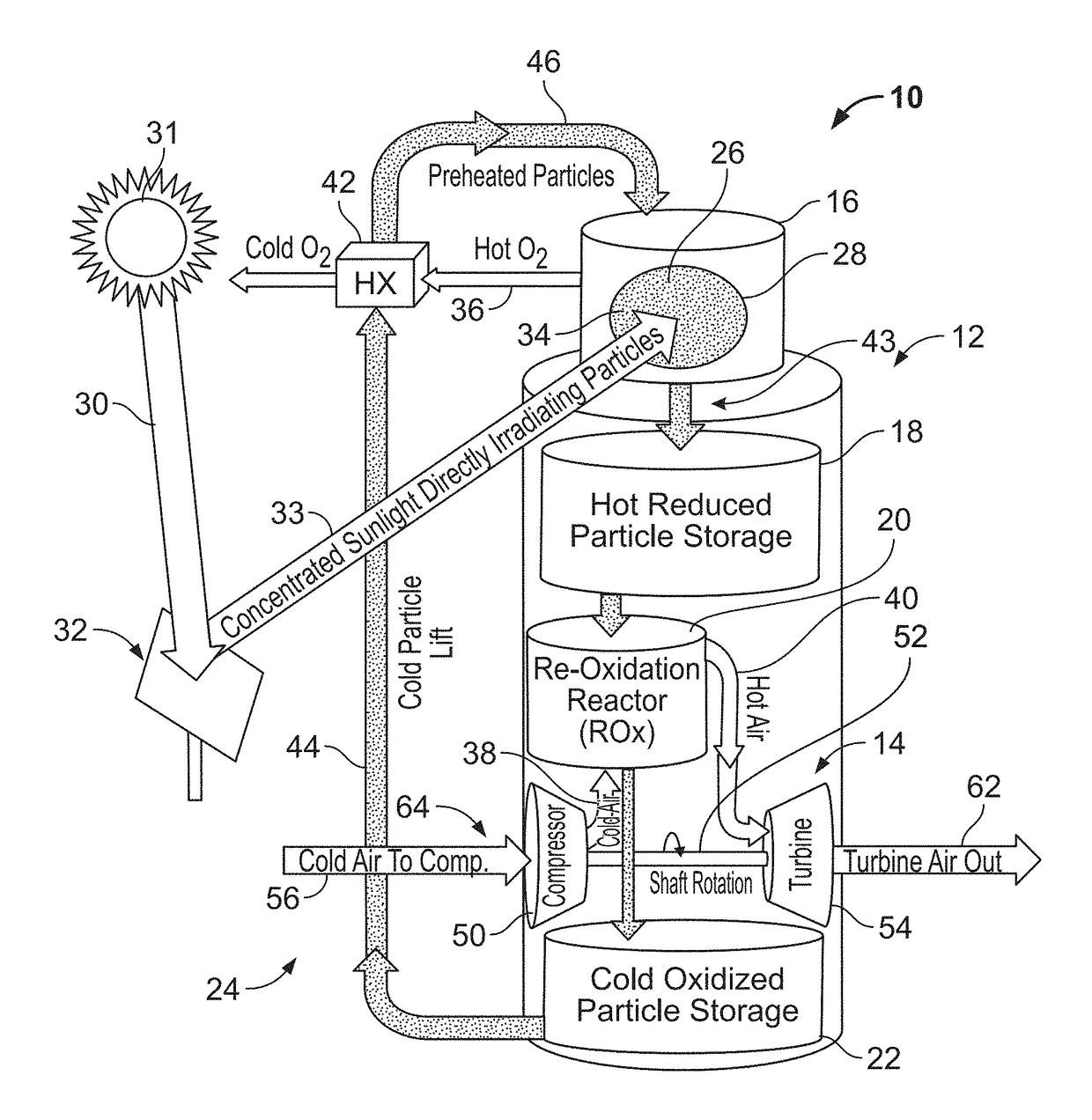
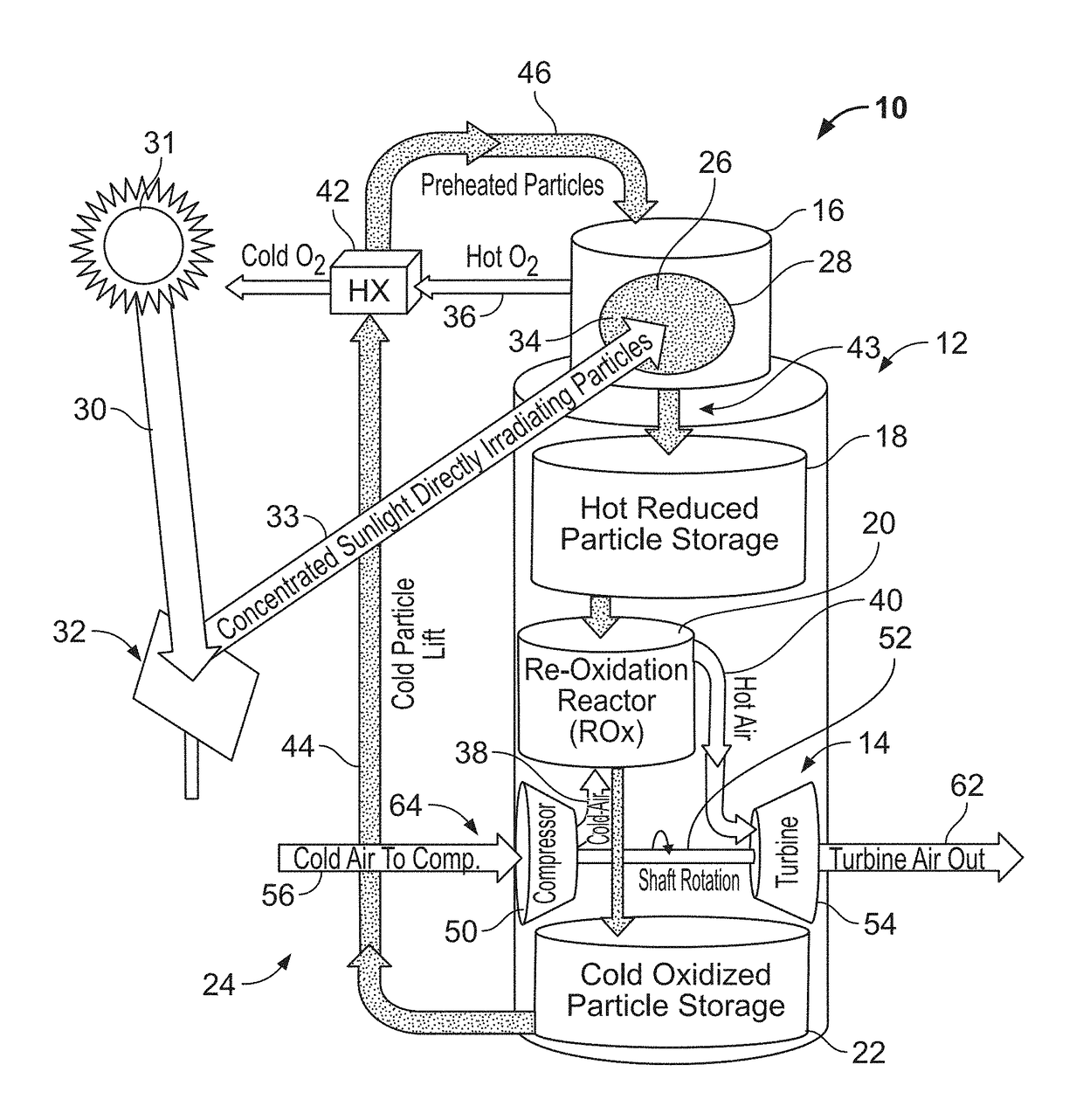
ActiveUS10107268B1Improve storage densityReduced levelized costFrom solar energyHeat storage plantsSeasonal thermal energy storageLattice oxygen
A solar power system and materials capable of storing heat energy by thermochemical energy storage are disclosed. Thermal energy is stored as chemical potential in these materials through a reversible reduction-oxidation reaction. Thermal energy from concentrated sunlight drives a highly endothermic reduction reaction that liberates lattice oxygen from the oxide to form O2 gas, leaving energy-rich, oxygen-depleted particles. When desired, the heat is recovered as the particles are re-oxidized in an exothermic reaction upon exposure to air. The system may be integrated with a power generation system to generate power.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com