Molybdenum-vanadium double-metal oxide catalyst and application of same to chemical-chain dehydrogenation of light alkanes
A double metal oxide and catalyst technology, applied in the field of oxidative dehydrogenation of low-carbon alkanes to olefins, supported molybdenum vanadium double metal oxides, to achieve the effect of increased conversion rate, high single-pass conversion rate, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Step 1, get the ammonium metavanadate (NH 4 VO 3 ) and 2.9 mass parts of oxalic acid (C 2 h 2 o 4 ) was dissolved in 3mL deionized water, after the reaction was complete, a certain mass of ammonium molybdate ((MH 4 ) 6 Mo 7 o 24 ﹒ 4H 2 O), the Al of 2.0 mass parts 2 o 3 Immerse in the above solution.
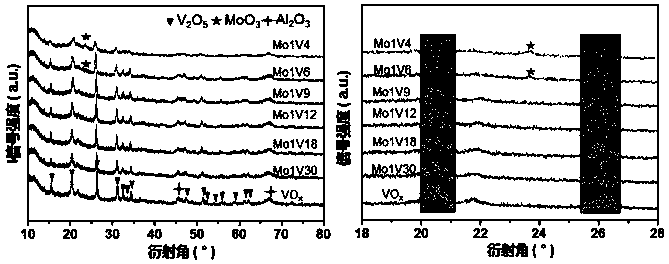
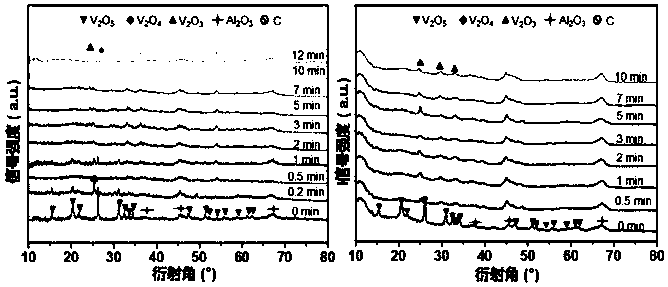
[0046] Step 2, drying the material obtained in step 1 at a room temperature of 25 degrees Celsius for 12 hours, then drying at 70 degrees Celsius for 12 hours, and finally roasting at 600 degrees Celsius for 4 hours in an air atmosphere to obtain a molybdenum-vanadium bimetallic composite oxide supported on alumina, Its molecular formula is Mo 1 V y . Wherein, y represents the amount of V relative to 1 mol of Mo, and y=4, 6, 9, 12, 18, 30, that is, the molar ratio of metal V to metal Mo.
[0047] Step 3, the Mo 1 V y The solid powder is compressed into tablets to make a granular catalyst with a size of 20-40 mesh.
Embodiment 2
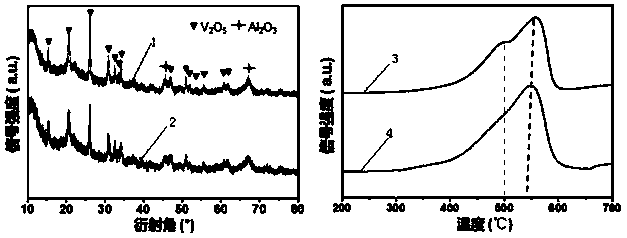
[0049] Adopt embodiment 1 method to react, its difference is only that the quality of the ammonium molybdate of step (1) is 0, obtains VO x catalyst.
Embodiment 3
[0051] Step 1, get the ammonium molybdate ((MH 4 ) 6 Mo 7 o 24 ﹒ 4H 2 O), be dissolved in 3mL deionized water, with 2.0 parts by mass of Al 2 o 3 Soak in the above solution, dry at room temperature for 12 hours, and dry at 80°C for 2 hours;
[0052] Step 2, drying the material obtained in step 1 at room temperature 25 degrees Celsius for 12 hours, then drying at 70 degrees Celsius for 12 hours, and finally roasting at 600 degrees Celsius for 4 hours in an air atmosphere to obtain molybdenum oxide supported on alumina, and its molecular formula is MoO x .
[0053] Step 3, the MoO x The solid powder is compressed into tablets to make a granular catalyst with a size of 20-40 mesh.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com