Low-carbon alkane chemical chain oxydehydrogenation to olefin technology
A carbon alkane chemical and oxidative dehydrogenation technology, applied in the direction of physical/chemical process catalysts, organic chemistry, chemical recovery, etc., can solve the problems of product cooling, high separation cost, decreased olefin selectivity, and difficult separation of by-products, etc., to achieve The effect of reducing catalyst carbon deposition, eliminating potential safety hazards, and reducing reaction energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
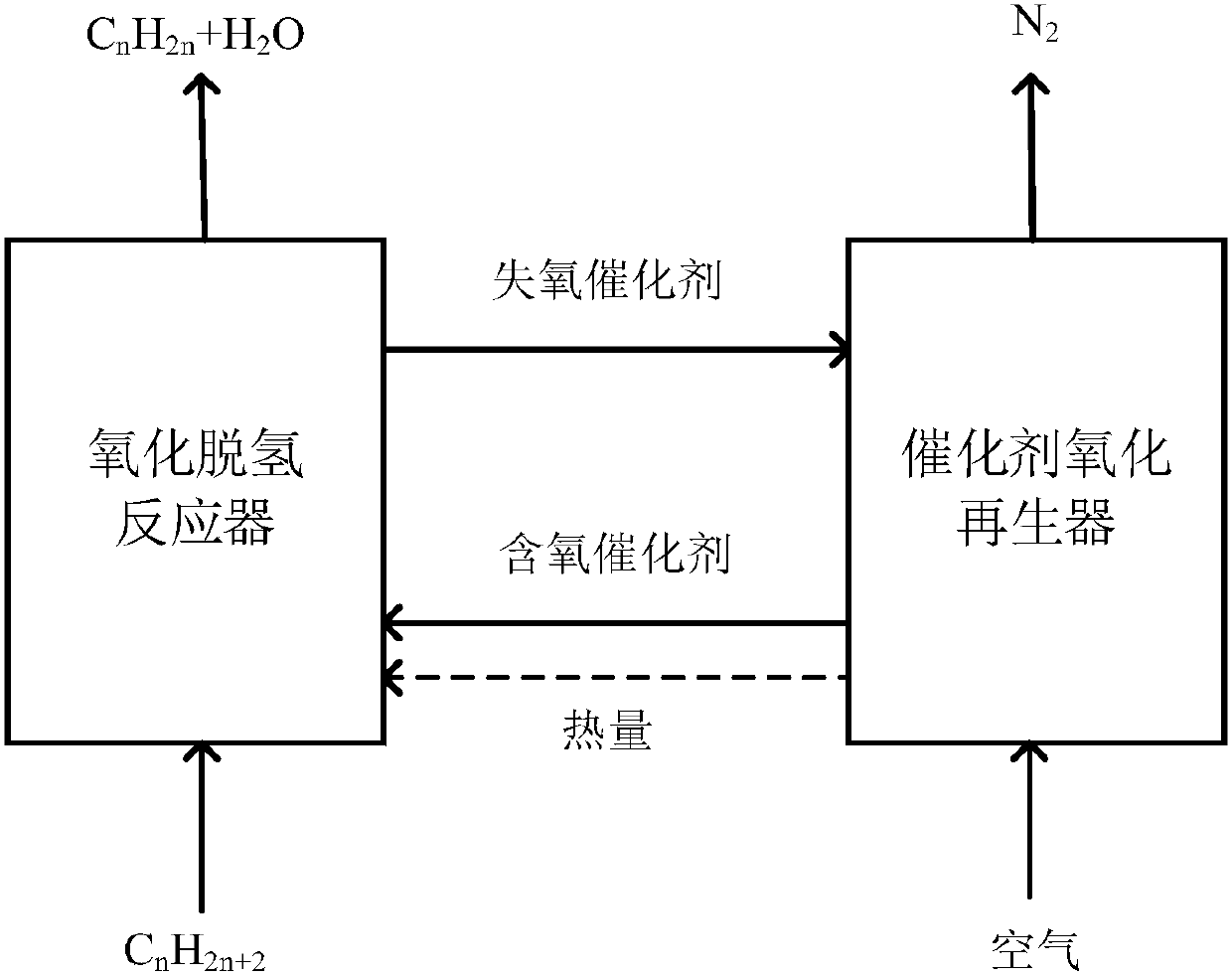
[0027] Such as figure 2 Shown are two side-by-side fixed bed propane chemical looping oxidative dehydrogenation to propylene systems. Compared with the reactor in which the catalyst particles are in a flowing state, the catalyst in the fixed bed reactor is not easy to lose, the gas flow is similar to plug flow, and the residence time of the reactants can be strictly controlled, and it is easy to obtain high conversion and selectivity. The reaction system includes:
[0028] Chemical chain oxidation dehydrogenation reactor 1: it has a propane raw material gas inlet, a reaction product outlet and a catalyst inlet. The propane raw material gas 6 is a mixture of 25% propane and 75% nitrogen, all of which are volume fractions, and is passed through the raw material gas inlet. Dehydrogenation reactor 1, the catalyst is loaded into the bed of reactor 1, the temperature of reactor 1 is 550°C, the pressure is normal pressure, the reaction product 7 is obtained after the oxidative dehy...
example 2
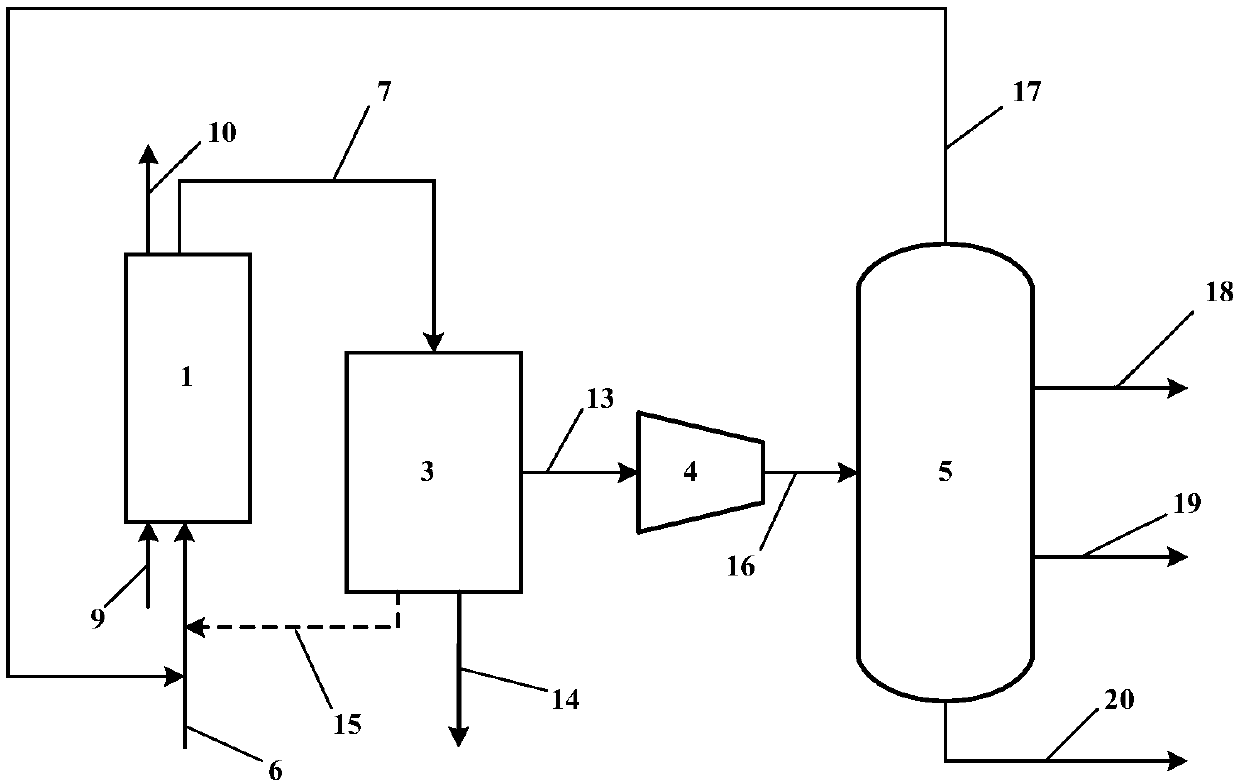
[0036] The process can also use a single fixed-bed reactor. in such as image 3 The shown single fixed-bed reactor unit carries out the propane chemical looping oxidative dehydrogenation to propylene process. This process is a batch operation process. The catalyst is loaded into the bed of the reactor 1, and the reaction raw material gas 6 is fed in, which is a mixed gas of 25% propane and 75% nitrogen, both in volume fraction. Reactor 1 temperature is 550 ℃, and pressure is normal pressure, and after reaction carries out 4h, catalyst loses activity, close propane inlet valve at this moment, feed a small amount of nitrogen to purge reaction gas in the device, close nitrogen inlet valve, Air 9 is introduced, and the catalyst is oxidized and regenerated at 550°C and atmospheric pressure. After regeneration, the oxygen-poor air 10 is discharged through the air outlet on the top of the reactor. The reaction time of oxidative regeneration is 1h. After the oxidation and regenerati...
example 3
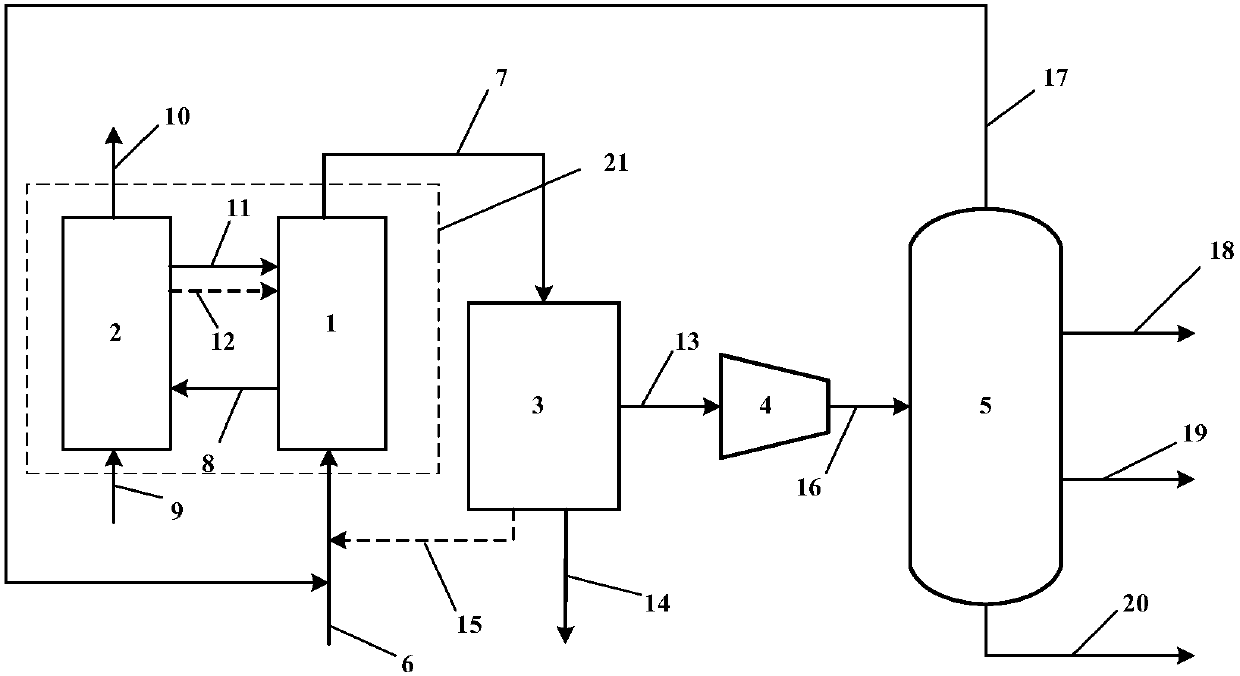
[0038] Figure 4 Shown is the process flow of chemical looping oxidative dehydrogenation of propane to propylene in a moving bed reactor device. The quenching unit 3, the compression unit 4, the separation unit 5 and the accompanying figure 2 Same process as shown, with Figure 4 will not be repeated. The reaction and regeneration process of the moving bed chemical chain oxidative dehydrogenation are carried out in the reactor and the regenerator respectively. The solid catalyst is continuously added from the top of the reactor, and as the reaction progresses, the solid catalyst gradually moves down by gravity, and finally is continuously discharged from the bottom. When a moving bed reactor is used, the solid-phase catalyst can move in the reactor and continuously enter and exit the reactor. The gas pressure drop is smaller than that of the fixed bed, and the back mixing is small. The solid residence time is between the fixed bed and the fluidized bed. and can vary over ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com