Calcium-titanium-ore type composite oxide La1-xSrxMO3-0.5 beta F beta
A composite oxide and perovskite-type technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of expanding reactor scale, high temperature gradient, Explosion hazard etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
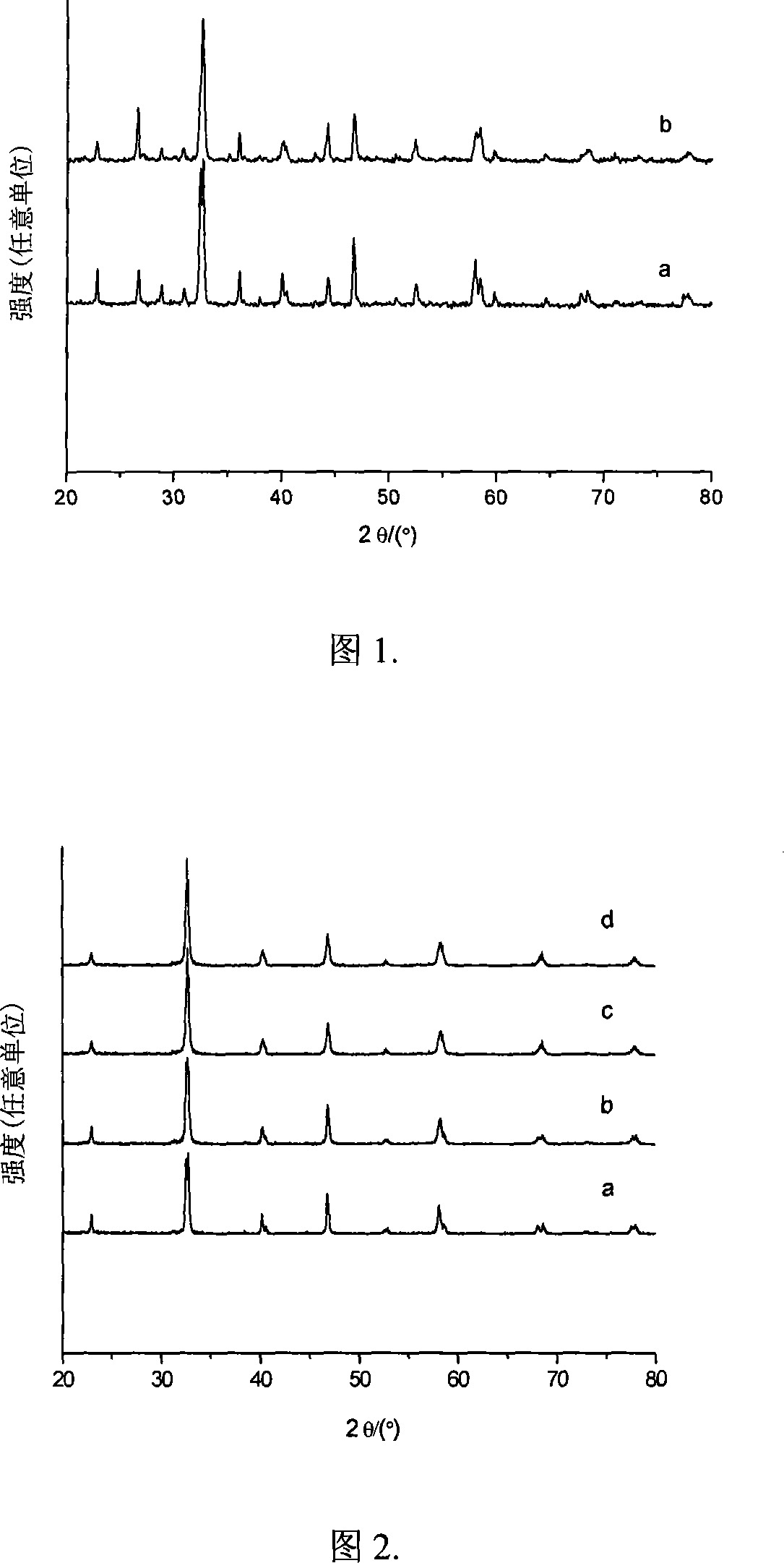
[0027] 5.09g La(NO3 ) 3 ·6H 2 O, 0.73g Sr(NO 3 ) 2 , 7.05g Fe(NO 3 ) 3 9H 2 O was dissolved in 100ml of distilled water to obtain a mixed solution. 7.87 g of glycine was added to the above mixed solution. The above solution was stirred continuously at 70°C and evaporated slowly until a viscous colloidal liquid was obtained. The colloidal liquid was heated in a muffle furnace at 250°C for 0.5 h to make it react quickly to obtain a powdery precursor. The precursor was calcined in air at 900 °C for 5 h to obtain the activated oxygen carrier La 0.8 Sr 0.2 FeO 3 . Sample specific surface area 6.1m 2 / g, the crystal phase contained is mainly a perovskite structure composite oxide (see Figure 1a).
[0028] The oxidation performance of the composite oxide of the present invention was tested by methane pulse reaction. In the methane pulse reaction, the carrier gas was argon with a flow rate of 23.5 ml / min. Pulse injection gas is CH 4 / Ar mixed gas, wherein the methane c...
Embodiment 2
[0030] 4.00g La(NO 3 ) 3 ·6H 2 O, 1.53g Sr(NO 3 ) 2 , 7.38g Fe(NO 3 ) 3 9H 2 O was dissolved in 100ml of distilled water to obtain a mixed solution. 7.95 g of glycine was added to the above mixed solution. The above solution was stirred continuously at 70°C and evaporated slowly until a viscous colloidal liquid was obtained. The colloidal liquid was heated in a muffle furnace at 250°C for 0.5 h to make it react quickly to obtain a powdery precursor. The precursor was calcined in air at 900 °C for 5 h to obtain the activated oxygen carrier La 0.6 Sr 0.4 FeO 3 . Sample specific surface area 6.9m 2 / g, the crystal phase contained is mainly a perovskite structure composite oxide (see Figure 1b).
[0031] The oxidation performance of the composite oxide of the present invention was tested by methane pulse reaction. In the methane pulse reaction, the carrier gas was argon with a flow rate of 23.5 ml / min. Pulse injection gas is CH 4 / Ar mixed gas, wherein the methane...
Embodiment 3
[0033] 8.67g La(NO 3 ) 3 2H 2 O, 1.27g Sr(NO 3 ) 2 , 10.74g 50%Mn(NO 3 ) 2 The solution was added to 100 ml of distilled water to obtain a mixed solution. 10.81 g of glycine was added to the above mixed solution. The above solution was stirred continuously at 70°C and evaporated slowly until a viscous colloidal liquid was obtained. The colloidal liquid was heated in a muffle furnace at 250°C for 0.5 h to make it react quickly to obtain a powdery precursor. The precursor was calcined in air at 900 °C for 5 h to obtain the activated oxygen carrier La 0.8 Sr 0.2 MnO 3 . Sample specific surface area 13.4m 2 / g, the contained crystal phase is a perovskite structure composite oxide (see Figure 2b).
[0034] The oxidation performance of the composite oxide of the present invention was tested by methane pulse reaction. In the methane pulse reaction, the carrier gas was argon with a flow rate of 23.5 ml / min. Pulse injection gas is CH 4 / Ar mixed gas, wherein the methane...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com