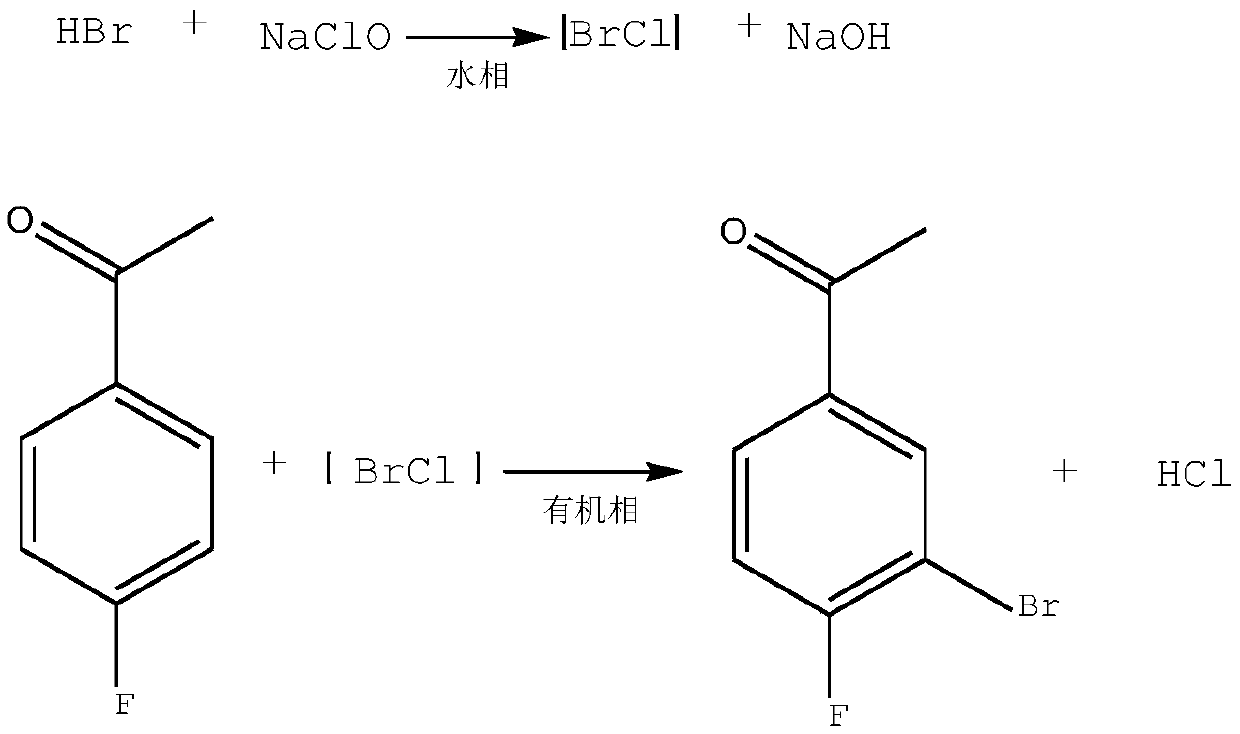
Synthesis method of 3-bromo-4-fluorobenzaldehyde
A technology of fluorobenzaldehyde and synthesis method, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of high environmental hazards and high risks, and achieve green processes, low risks, and high yields. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of synthetic method of 3-bromo-4-fluorobenzaldehyde, comprises the following steps:
[0026] (1) 1mol of 4-fluorobenzaldehyde was dissolved in 160mL of dichloromethane to obtain solution A;
[0027] (2) Dissolve 1.01 mol of sodium bromide in 100 mL of pure water, and add 100 mL of 35% hydrochloric acid under stirring to obtain solution B;
[0028] (3) At 20-25°C, after mixing solution A and solution B, turn on the ultrasonic wave, and add 1.02mol of 8% sodium hypochlorite aqueous solution dropwise within 1 hour under stirring;
[0029] (4) dropwise addition is completed, heat preservation 30 minutes under ultrasonic, stirring; Then stand still for 15 minutes;
[0030] (5) phase separation, dichloromethane phase washing to neutrality, drying and precipitation;
[0031] (6) The crude product was obtained, and the bulk melted and crystallized at 31°C to obtain the pure product.
[0032] Finally, 185 g of pure product was obtained, with a purity of 99.2% and a yie...
Embodiment 2
[0034] A kind of synthetic method of 3-bromo-4-fluorobenzaldehyde, comprises the following steps:
[0035] (1) 1mol of 4-fluorobenzaldehyde was dissolved in 140mL of dichloromethane to obtain solution A;
[0036] (2) Dissolve 1.03 mol of sodium bromide in 110 mL of pure water, and add 110 mL of 35% hydrochloric acid under stirring to obtain solution B;
[0037] (3) At 20-25°C, after mixing solution A and solution B, turn on the ultrasonic wave, and add 1.03mol 8% sodium hypochlorite aqueous solution dropwise within 1 hour under stirring;
[0038] (4) dropwise addition is completed, heat preservation 30 minutes under ultrasonic, stirring; Then stand still for 15 minutes;
[0039] (5) phase separation, dichloromethane phase washing to neutrality, drying and precipitation;
[0040] (6) The crude product was obtained, and the bulk melted and crystallized at 31°C to obtain the pure product.
[0041] Finally, 187.7 g of pure product was obtained, with a purity of 99.4% and a yiel...
Embodiment 3
[0043] A kind of synthetic method of 3-bromo-4-fluorobenzaldehyde, comprises the following steps:
[0044] (1) 1mol of 4-fluorobenzaldehyde was dissolved in 160mL of dichloromethane to obtain solution A;
[0045] (2) Dissolve 1 mol of sodium bromide in 90 mL of pure water, and add 90 mL of 35% hydrochloric acid under stirring to obtain solution B;
[0046] (3) At 20-25°C, after mixing solution A and solution B, turn on the ultrasonic wave, and add 1.04mol 8% sodium hypochlorite aqueous solution dropwise within 1 hour under stirring;
[0047] (4) dropwise addition is completed, heat preservation 30 minutes under ultrasonic, stirring; Then stand still for 15 minutes;
[0048] (5) phase separation, dichloromethane phase washing to neutrality, drying and precipitation;
[0049] (6) The crude product was obtained, and the bulk melted and crystallized at 31°C to obtain the pure product.
[0050] Finally, 183.6 g of pure product was obtained, with a purity of 99.2% and a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com