Organopolysiloxane compound and active energy ray-curable composition containing the same
A curable composition and technology of active energy rays, applied in the field of organopolysiloxane compounds and active energy ray curable compositions containing them, and active energy ray curable compositions, can solve the corrosion of metal wiring parts, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
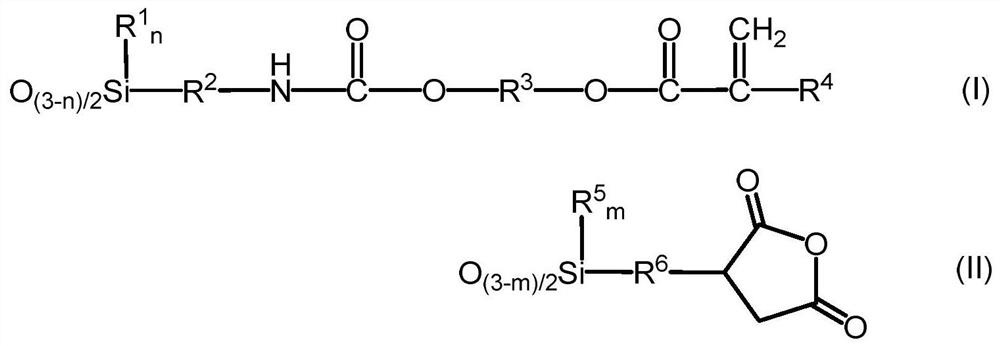
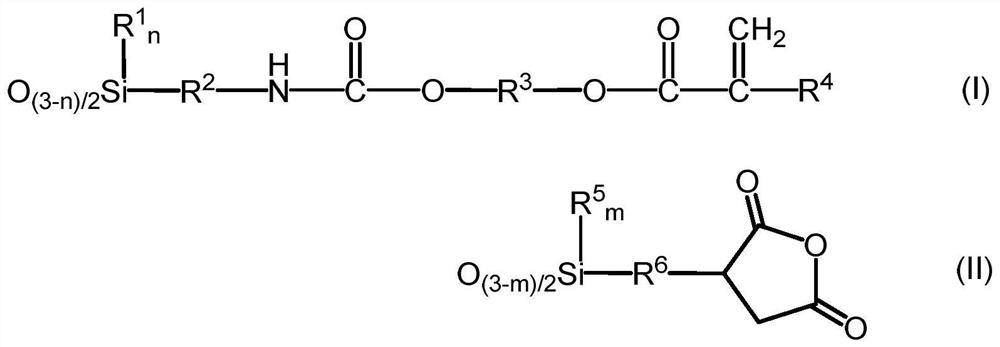
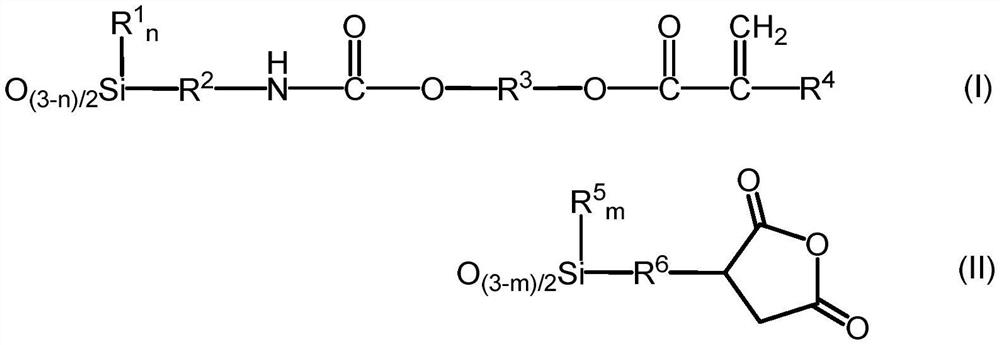
[0117] 3-Isocyanatopropyltrimethoxysilane (KBM-9007, manufactured by Shin-Etsu Chemical Co., Ltd.) was added while stirring 348.3 g (3.0 mol) of hydroxyethyl acrylate (manufactured by Osaka Organic Chemical Industry Co., Ltd.) in the reactor. After production) 615.9 g (3.0 mol), the mixture was stirred at 25° C. for 1 hour to obtain 964.2 g (3.0 mol) of a compound represented by the following formula (VI).
[0118] Thereto were added 262.3 g (1.0 mol) of 3-(trimethoxysilyl)propyl succinic anhydride, 487.1 g (3.0 mol) of hexamethyldisiloxane, and 7.2 g of methanesulfonic acid, and added when it became uniform. 147.6 g of ion-exchanged water was stirred at 25°C for 4 hours. 35.9 g of Kaiwa Chemical Industry Co., Ltd. 500SH (manufactured by Kyowa Chemical Industry Co., Ltd.) was put in, stirred for 2 hours, and neutralized. Volatile components such as methanol were distilled off under reduced pressure, followed by pressure filtration.
[0119] The obtained reactant was a viscou...
Embodiment 1-2
[0123] 642.8 g (2.0 mol) of the compound represented by the above formula (VI), 524.7 g (2.0 mol) of 3-(trimethoxysilyl)propylsuccinic anhydride, 487.1 g (3.0 mol) of hexamethyldisiloxane , 6.9 g of methanesulfonic acid was put into the reactor, 165.6 g of ion-exchanged water was added when it became uniform, and the mixture was stirred at 25° C. for 2 hours. 34.5 g of Kaiwa Chemical Industry Co., Ltd. 500SH (manufactured by Kyowa Chemical Industry Co., Ltd.) was put in, stirred for 2 hours, and neutralized. Volatile components such as methanol were distilled off under reduced pressure, followed by pressure filtration.
[0124] The obtained reactant was a viscous liquid at 25°C having a viscosity of 3200 mPa·s at 25°C, a volatile content of 2.5% by mass, and a weight average molecular weight of 1530. The values of a to g in the average formula (IV) calculated from the results of NMR were a=0.22, b=0.23, c=0, d=0, e=0, f=0.55, and g=0.02, respectively.
Embodiment 1-3
[0126] 642.8 g (2.0 mol) of the compound represented by the above formula (VI), 262.3 g (1.0 mol) of 3-(trimethoxysilyl)propylsuccinic anhydride, 136.2 g (1.0 mol) of methyltrimethoxysilane, 487.1 g (3.0 mol) of hexamethyldisiloxane and 6.3 g of methanesulfonic acid were put into the reactor, and 147.6 g of ion-exchanged water was added when it became uniform, and the mixture was stirred at 25° C. for 2 hours. 31.3 g of Kaiwa Chemical Industry Co., Ltd. 500SH (manufactured by Kyowa Chemical Industry Co., Ltd.) was put in, stirred for 2 hours, and neutralized. Volatile components such as methanol were distilled off under reduced pressure, followed by pressure filtration.
[0127] The obtained reactant was a viscous liquid at 25°C having a viscosity of 2990 mPa·s at 25°C, a volatile content of 2.1% by mass, and a weight average molecular weight of 1770. The values of a to g in the average formula (IV) calculated from the results of NMR were a=0.26, b=0.09, c=0, d=0.10, e=0, f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com