Organopolysiloxane compound and active energy ray curable composition containing the same
A curable composition and active energy ray technology, applied in organic chemistry, compounds of group 4/14 elements of the periodic table, chemical instruments and methods, etc., can solve problems such as corrosion of metal wiring parts and changes over time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
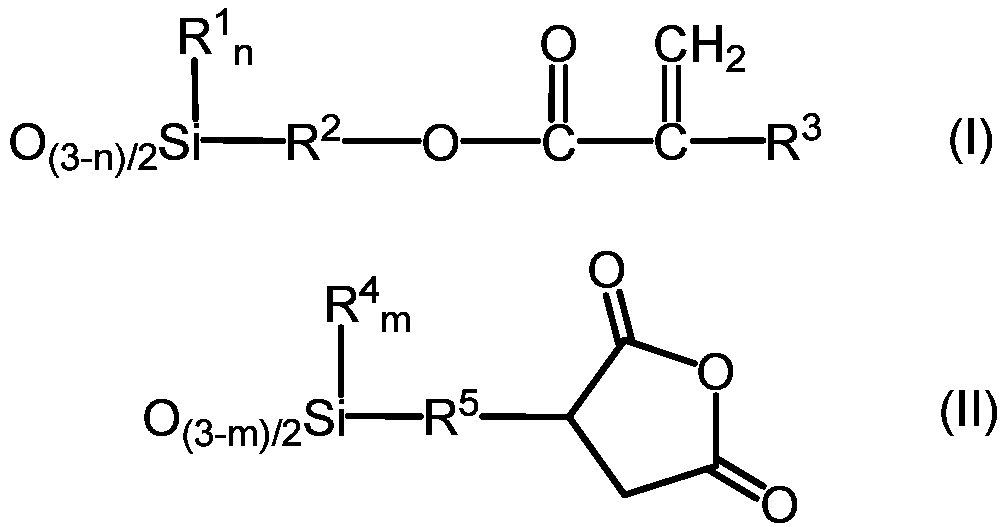
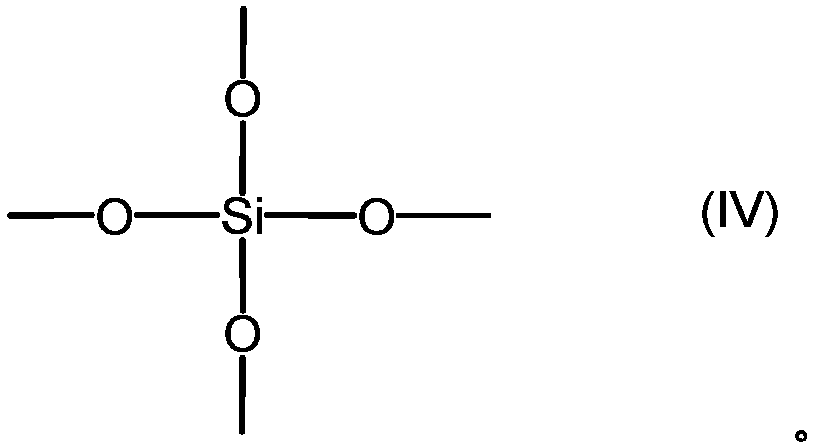
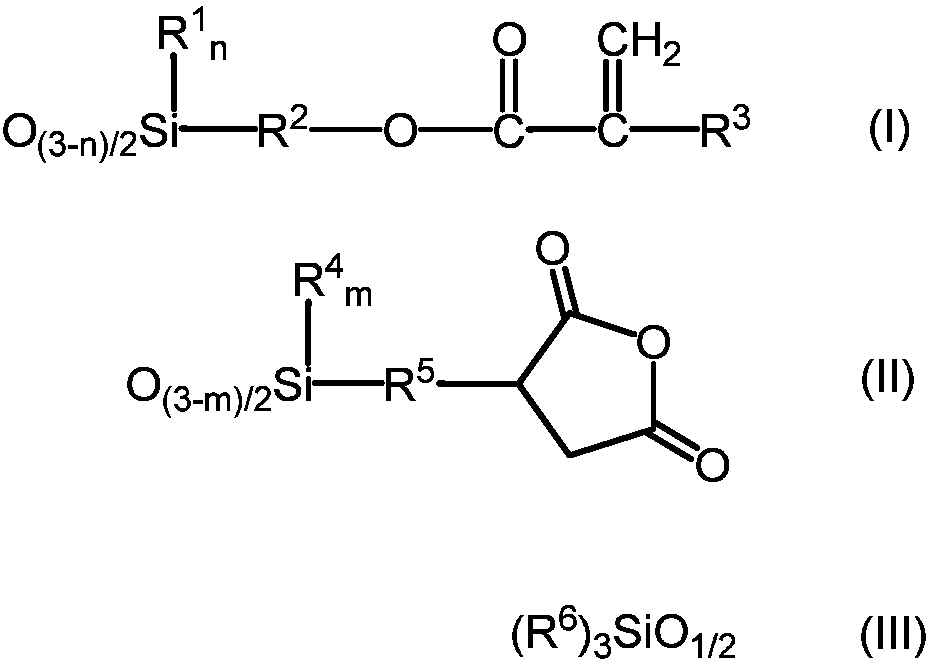
[0112] 468.6g (2.0mol) of 3-acryloxypropyltrimethoxysilane, 262.3g (1.0mol) of 3-(trimethoxysilyl)propylsuccinic anhydride, 487.13g of hexamethyldisiloxane (3.0 mol) and 5.1 g of methanesulfonic acid were added to the reactor, and when it became uniform, 115.2 g of ion-exchanged water was added, and stirred at 25° C. for 4 hours. 25.3 g of Keyword 500SH (manufactured by Kyowa Chemical Industry Co., Ltd.) was charged, stirred for 2 hours, and neutralized. Volatile components such as methanol were distilled off under reduced pressure, and pressure filtration was performed.
[0113] The obtained reactant was a liquid having a viscosity of 890 mPa·s at 25° C., a volatile component of 6.4% by mass, and a weight average molecular weight of 1180. The values of a to g in the average formula (V) calculated from the results of NMR were a=0.29, b=0.13, c=0, d=0, e=0, f=0.58, and g=0.13, respectively.
Embodiment 1-2
[0115] 468.6g (2.0mol) of 3-acryloxypropyltrimethoxysilane, 524.7g (2.0mol) of 3-(trimethoxysilyl)propylsuccinic anhydride, 487.1g of hexamethyldisiloxane (3.0 mol) and 6.0 g of methanesulfonic acid were added to the reactor, and when it became uniform, 165.6 g of ion-exchanged water was added, and stirred at 25° C. for 2 hours. 30.1 g of Keyword 500SH (manufactured by Kyowa Chemical Industry Co., Ltd.) was added, stirred for 2 hours, and neutralized. Volatile components such as methanol were distilled off under reduced pressure, and pressure filtration was performed.
[0116] The obtained reactant was a liquid having a viscosity of 550 mPa·s at 25° C., a volatile content of 3.3% by mass, and a weight average molecular weight of 970. The values of a to g in the average formula (V) calculated from the results of NMR were a=0.26, b=0.26, c=0, d=0, e=0, f=0.48, g=0.11, respectively.
Embodiment 1-3
[0118] 702.9g (3.0mol) of 3-acryloxypropyltrimethoxysilane, 262.3g (1.0mol) of 3-(trimethoxysilyl)propylsuccinic anhydride, 487.1g of hexamethyldisiloxane (3.0 mol) and 5.1 g of methanesulfonic acid were charged into the reactor, and when it became uniform, 147.6 g of ion-exchanged water was added, and stirred at 25° C. for 4 hours. 25.3 g of Keyword 500SH (manufactured by Kyowa Chemical Industry Co., Ltd.) was charged, stirred for 2 hours, and neutralized. Volatile components such as methanol were distilled off under reduced pressure, and pressure filtration was performed.
[0119] The obtained reactant was a liquid having a viscosity of 190 mPa·s at 25° C., a volatile content of 4.1% by mass, and a weight average molecular weight of 1,210. The values of a to g in the average formula (V) calculated from the results of NMR were a=0.42, b=0.14, c=0, d=0, e=0, f=0.44, g=0.08, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com