Bio-based photo-curable polyurethane and photoresist prepared with same
A technology of photocuring and polyurethane, applied in the field of photoresist, to achieve the effect of increasing the glass transition temperature, good comprehensive performance and high resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of bio-based photocurable polyurethane:
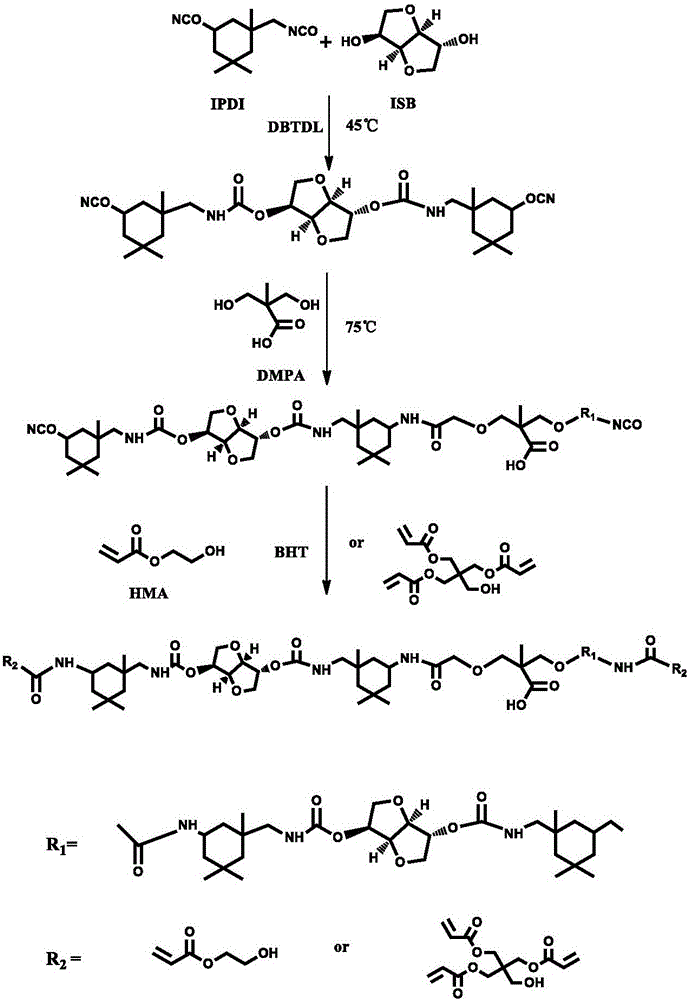
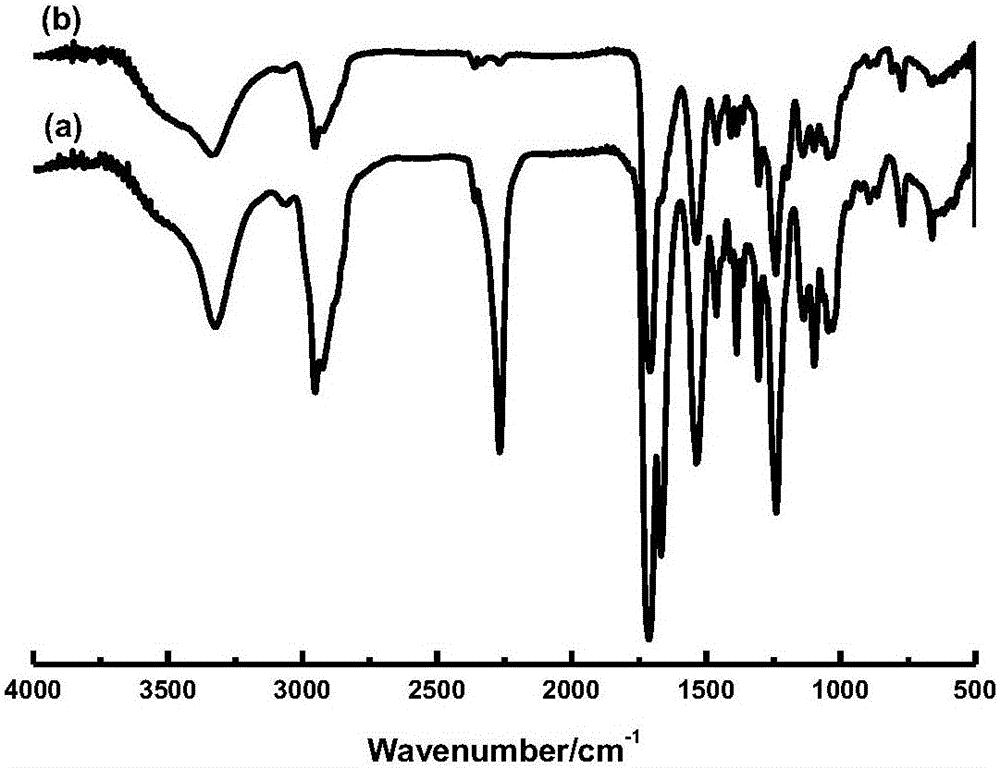
[0032] Add 44.458 g of isophorone diisocyanate (IPDI) and 0.295 g of dibutyltin dilaurate (DBTDL) into a 250 mL four-necked round bottom flask equipped with a thermometer, a reflux condenser, and a stirrer, and drop isosorbide into it (ISB) 14.614g, add 15g of N,N-dimethylformamide to adjust the viscosity of the system, keep the reaction at 45°C for 2h, then add 6.706g of 2,2-dimethylolpropionic acid (DMPA), Insulation reaction for 4 hours, finally add 0.420g of polymerization inhibitor 2,6-di-tert-butyl-p-cresol, and then add 13.934g of hydroxyethyl acrylate (HEA), until NCO can not be detected by infrared spectroscopy at 2265cm -1 When the absorption peak at figure 2 As shown, stop the reaction, the required time is 5h, and finally obtain bio-based photocurable polyurethane.
[0033] The synthetic structural route of the obtained bio-based photocurable polyurethane is shown in figure 1 shown. The whole pro...
Embodiment 2
[0038] (1) Preparation of bio-based photocurable polyurethane:
[0039] Add 44.458 g of isophorone diisocyanate (IPDI) and 0.295 g of dibutyltin dilaurate (DBTDL) into a 250 mL four-necked round bottom flask equipped with a thermometer, a reflux condenser, and a stirrer, and drop isosorbide into it (ISB) 14.614g, add 15g of N,N-dimethylformamide to adjust the viscosity of the system, keep the reaction at 45°C for 2h, then add 6.706g of 2,2-dimethylolpropionic acid (DMPA), Insulation reaction for 4h, finally add 0.420g of polymerization inhibitor 2,6-di-tert-butyl-p-cresol, and then add 55.795g of pentaerythritol triacrylate (PETA), until NCO cannot be detected by infrared spectroscopy at 2265cm -1 When the absorption peak at the place, stop the reaction, the required time is 5h, and finally obtain the bio-based photocurable polyurethane.
[0040] The whole process measures the NCO group content during the reaction by the toluene-di-n-butylamine back titration method, and proc...
Embodiment 3
[0045] (1) Preparation of bio-based photocurable polyurethane:
[0046] Add 44.458 g of isophorone diisocyanate (IPDI) and 0.295 g of dibutyltin dilaurate (DBTDL) into a 250 mL four-necked round-bottomed flask equipped with a thermometer, a reflux condenser, and a stirrer, and drop isosorbide Alcohol (ISB) 14.614g, add 15g of N,N-dimethylformamide to adjust the viscosity of the system, keep the reaction at 45°C for 2h, then add 6.706g of 2,2-dimethylolpropionic acid (DMPA), in 75 ℃ heat preservation reaction for 4h, finally add 0.420g of polymerization inhibitor 2,6-di-tert-butyl p-cresol, then add 13.934g of hydroxyethyl acrylate (HEA), until NCO can not be detected by infrared spectroscopy at 2265cm -1 When the absorption peak at the place, stop the reaction, the required time is 5h, and finally obtain the bio-based photocurable polyurethane.
[0047] The whole process measures the NCO group content during the reaction by the toluene-di-n-butylamine back titration method, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com