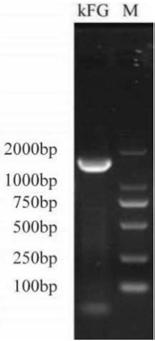
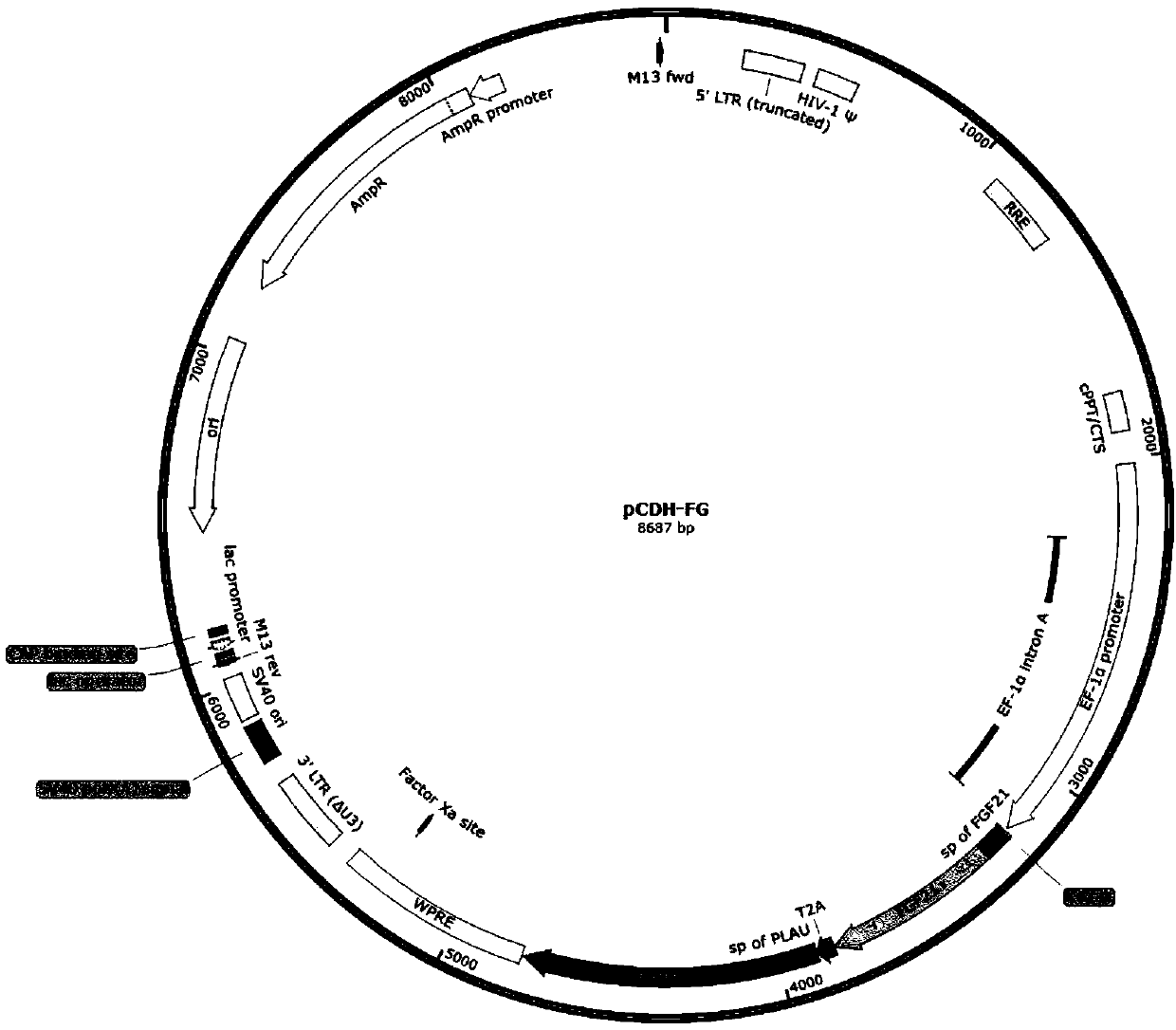
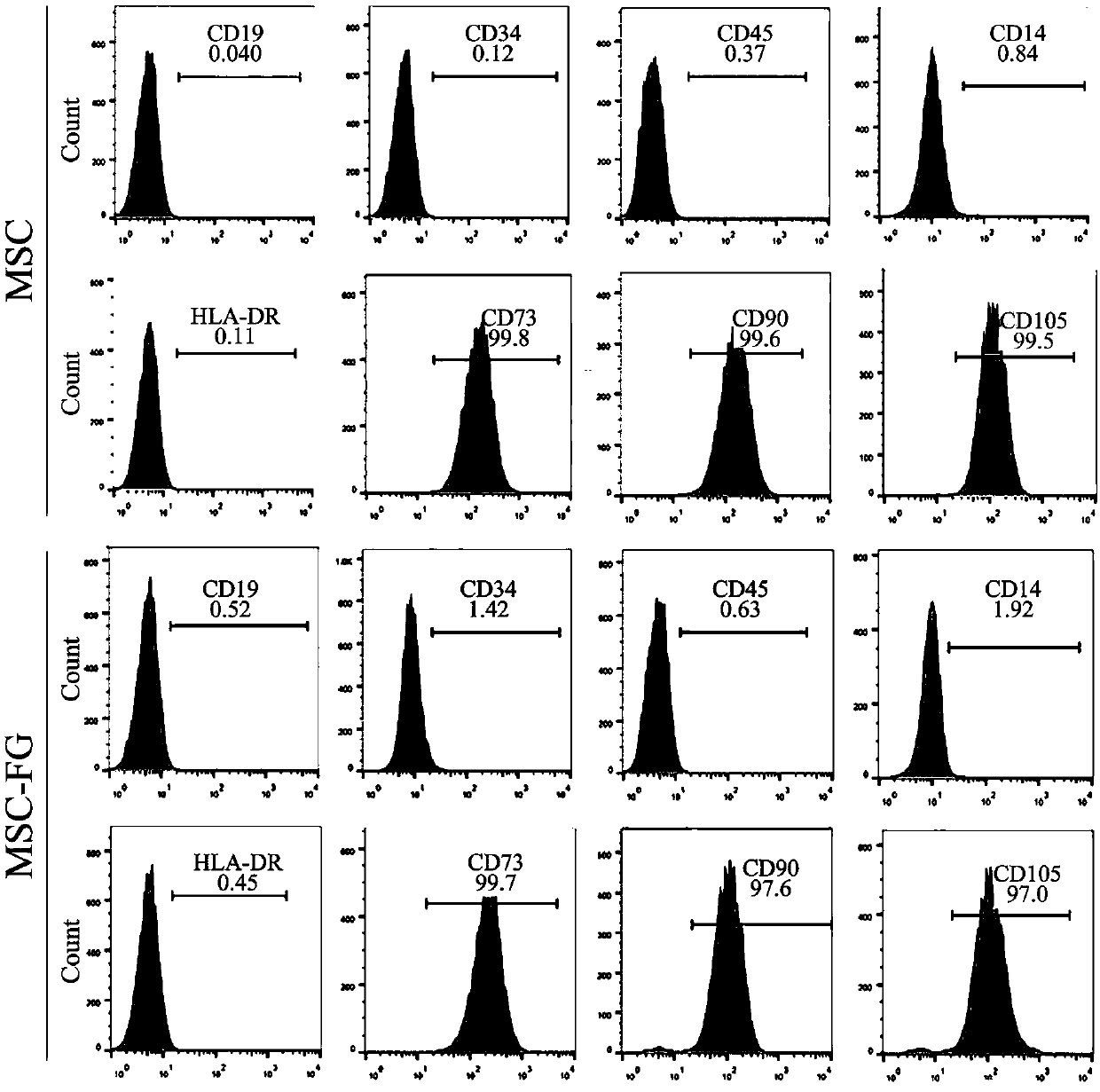
Double-gene modified stem cell and use thereof
A technology of mesenchymal stem cells and amino acids, which is applied in the preparation of drugs for the treatment of metabolic diseases in subjects, in the field of treatment of metabolic diseases, and can solve the problems of not being able to fundamentally improve the cause of the disease, long-term medication, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] Example 1. Preparation of FGF21 / GLP1-Fc Modified Mesenchymal Stem Cells
[0159] 1.1 Isolation and culture of autologous adipose-derived stem cells
[0160] Adipose derived mesenchymal stem cells (AD-MSCs) were isolated and cultured by mixed collagenase digestion method, the specific method is as follows:
[0161] Transfer the healthy adult adipose tissue extracted by liposuction to a 50mL centrifuge tube, add PBS for sufficient washing, and centrifuge at 1500rpm for 5 minutes to obtain the upper layer of adipose tissue. According to the ratio of 1:1:1, type I, II and IV collagenases were mixed to prepare 0.2% mixed collagenase, and the adipose tissue was added into the mixed collagenase digestion solution according to the ratio of adipose tissue: collagenase=1:1, Digest adipose tissue in a shaker at 37°C for 30 minutes. The digested adipose tissue was immediately added to 10% FBS α-MEM cell culture medium (purchased from Gibco), centrifuged at 1500 rpm for 10 minut...
Embodiment 2
[0177] Example 2. In vitro biological activity evaluation of FGF21 / GLP1-Fc modified mesenchymal stem cells
[0178] 2.1 Effect of MSC-FG on glucose-stimulated insulin secretion
[0179] Pre-preparation: Culture MSC, MSC-FG, MSC-FGF21 and MSC-GLP1-Fc cells obtained in Example 1 respectively in 100mm culture, when the confluence of the cells reaches 70%-80%, discard the original MSC serum-free medium, 10ml α-MEM medium, 37°C, 5% CO 2 Saturated humidity continued to cultivate for 48h. The culture supernatants of the four kinds of cells were collected, concentrated 10 times with an ultrafiltration column, and stored at 4°C for future use. Long-term storage needs to be placed in a -80°C refrigerator.
[0180] Take out a tube of frozen INS-1 cells (rat insulinoma cells, donated by the Academy of Military Medical Sciences) from liquid nitrogen and quickly put it in a 37°C water bath until the ice cubes disappear, and add 5 ml of preheated medium dropwise In a 15ml centrifuge tu...
Embodiment 3
[0189] Example 3. In vivo biological activity evaluation of FGF21 / GLP1-Fc modified mesenchymal stem cells
[0190] 3.1 Experimental grouping
[0191] Thirty 5-week-old male diabetic model mice BKS.Cg-Dock7m+ / +Leprdb / Nju (purchased from the Institute of Model Animals, Nanjing University) were selected. The experiment was divided into: control group (normal saline), liraglutide drug group (drug), MSC group (cell), MSC-FGF21 group (cell), MSC-FG group (cell) and MSC-GLP1-Fc group ( cells), a total of 6 groups, 5 mice in each group.
[0192] 3.2 Treatment plan
[0193] The administration method of liraglutide drug group (Liraglutide) was subcutaneous injection, and the administration method of the other groups was intraperitoneal injection, and all groups required administration in the morning.
[0194] Dosing time: The cell group requires that the cells be injected every 7 days, and the cells are given 3 times in total. The liraglutide drug group required the drug to be given t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com