Preparation method for 2-(alpha-hydroxyl aryl) benzimidazole compound
A benzimidazole and hydroxyaryl technology, applied in the field of organic compound synthesis, can solve the problems of high reaction temperature, poor substrate universality, and low yield, and achieve good substrate universality, mild reaction conditions, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of 1-tert-butoxycarbonyl-2-(α-hydroxyphenyl)benzimidazole, the structural formula is as follows:
[0025] .
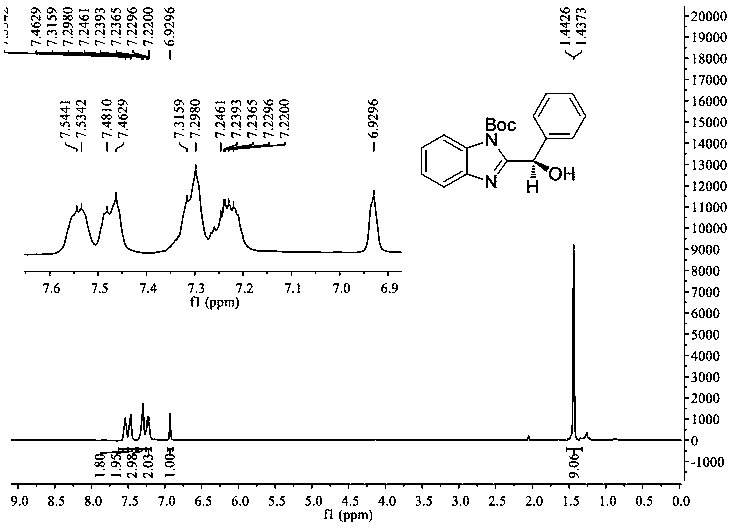
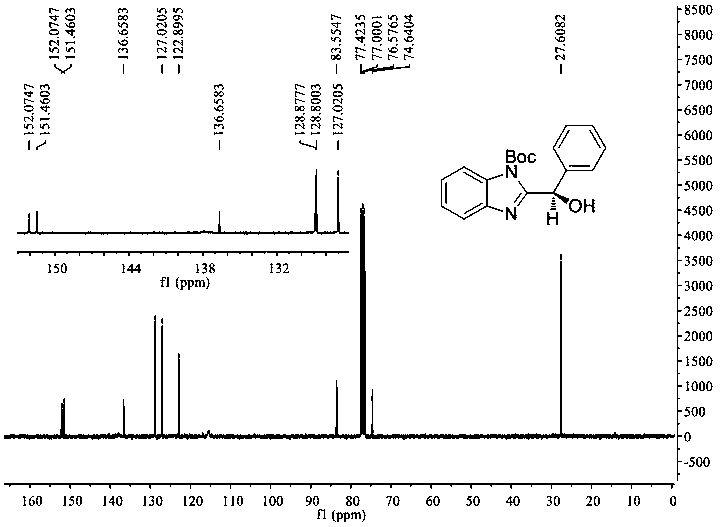
[0026] Preparation: In a dry 25 mL schlenk tube, add 1-tert-butoxycarbonyl-2-benzoyl-benzimidazole (0.097 g, 0.3 mmol), photosensitizer (0.00053 g, 0.0015 mmol), N-phenylpiperene Pyridine (0.097 g, 0.6 mmol) and chiral spirocyclic phosphoric acid catalyst (0.028 g, 0.03 mmol), continue to add 5 mL of cyclopentyl methyl ether, and degas with a vacuum pump for 3 times at -78 ° C, 5 min each time , and then placed at -30°C and irradiated with a 3W blue light for 48 hours. After complete reaction, separate by column chromatography (petroleum ether / ethyl acetate = 20 / 1 ~ 8 / 1), concentrate by rotary evaporation, and dry in vacuum (at 25°C for 1 hour) to obtain 1-tert-butoxycarbonyl- 2-(α-Hydroxyphenyl)benzimidazole (0.079 g, 0.243 mmol), yield 81%. The detection results of its hydrogen spectrum and carbon spectrum are as follows (see figur...
Embodiment 2
[0034] Example 2 Preparation of 1-tert-butoxycarbonyl-2-(α-hydroxy-4-methylphenyl)benzimidazole, the structural formula is as follows:
[0035] .
[0036] Preparation process: Add 1-tert-butoxycarbonyl-2-(4-methylbenzoyl)-benzimidazole (0.1 g, 0.3 mmol), photosensitizer (0.00053 g, 0.0015 mmol) into a dry 25 mL schlenk tube ), N-phenylpiperidine (0.097g, 0.6 mmol) and chiral spirocyclic phosphoric acid catalyst (0.028 g, 0.03 mmol), continue to add 5 mL of cyclopentyl methyl ether, and degas with a vacuum pump at -78 °C 3 times, 5min each time, then placed at -30°C and irradiated with a 3W blue light for 48 hours. After complete reaction, separate by column chromatography (petroleum ether / ethyl acetate = 20 / 1 ~ 8 / 1), concentrate by rotary evaporation, and dry in vacuum (at 25°C for 1 hour) to obtain 1-tert-butoxycarbonyl -2-(α-Hydroxy-4-methylphenyl)benzimidazole (0.087 g, 0.258 mmol), yield 86%, enantiomeric excess 90%. The spectrum detection results are as follows:
[...
Embodiment 3
[0038] Example 3 Preparation of 1-tert-butoxycarbonyl-2-(α-hydroxy-4-fluorophenyl)benzimidazole, the structural formula is as follows:
[0039] .
[0040] Preparation process: Add 1-tert-butoxycarbonyl-2-(4-fluorobenzoyl)-benzimidazole (0.1 g, 0.3 mmol), photosensitizer (0.00053 g, 0.0015 mmol) into a dry 25 mL schlenk tube, N-phenylpiperidine (0.097 g, 0.6 mmol) and chiral spirocyclic phosphoric acid catalyst (0.028 g, 0.03 mmol), continue to add 5 mL of cyclopentyl methyl ether, and degas with a vacuum pump at -78 °C for 3 time, 5min each time, and then placed at -30°C and irradiated with a 3W blue light for 48 hours. After complete reaction, separate by column chromatography (petroleum ether / ethyl acetate = 20 / 1 ~ 8 / 1), concentrate by rotary evaporation, and dry in vacuum (at 25°C for 1 hour) to obtain 1-tert-butoxycarbonyl -2-(α-Hydroxy-4-fluorophenyl)benzimidazole (0.075 g, 0.228 mmol), yield 76%, enantiomeric excess 90%. The spectrum detection results are as follows...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com