A kind of chitinase low temperature resistant mutant and its application
A technology of chitinase and chitin, which is applied in the direction of application, enzyme, and the introduction of foreign genetic material by using vectors, etc., can solve the problem that there are not many low-temperature chitinases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]Example 1 Gene cloning of wild-type chitinase
[0024] The present inventors have discovered a chitinase screened from Pseudoalteromonas sp. amino acid sequence as shown in SEQ ID NO: 1 disclosed in the prior art, the nucleotide encoding the chitinase as shown in SEQ ID NO: 1 ID NO: 2, expressed in E. coli. In short, the gene was artificially synthesized by Shanghai Jierui Biotechnology Co., Ltd., and then ligated into the pET-28(+) vector (New England Biolabs) between the restriction sites of EcoR I and HindIII, and transformed into the large intestine. Bacillus DH5α was screened on LB plates containing 50 mg / L ampicillin, and the expression vector contained in the screened positive bacteria was named pET-chi. The plasmid was extracted and sequenced and identified correctly.
Embodiment 2
[0025] Example 2 Mutation Screening
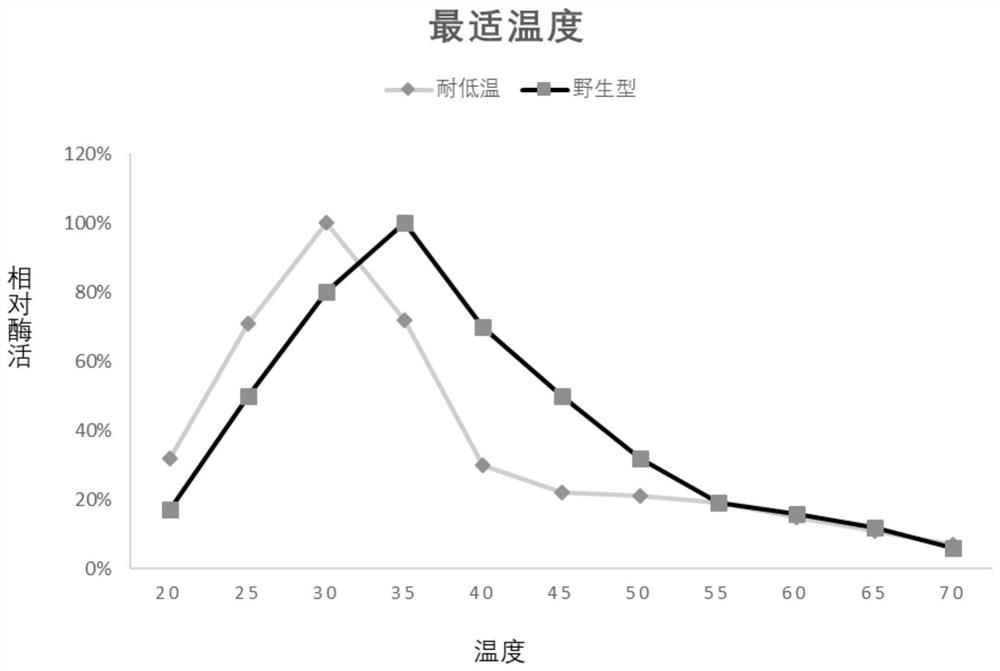
[0026] In order to further improve the low temperature resistance of the above-mentioned chitinase (amino acid sequence is SEQ ID NO: 1), the applicant used the recombinant plasmid pET-chi constructed in Example 1 as a template, designed random mutation primers respectively, and carried out error-prone primers. PCR, multi-point random mutation of the target gene, it was found that some mutations have no effect on its low temperature resistance, some mutations even make the low temperature resistance worse, and some mutations, although they can improve heat resistance, but after mutation The applicable range of pH is narrowed, and none of them meet the requirements. Finally, the applicant obtained a combination of mutation sites that can not only significantly improve low temperature performance, but also expand the scope of application under acidic conditions: R501L, Y555W, F635P, A673S, E689T; specifically, Arg at position 501 is mutated ...
Embodiment 3
[0029] Example 3 Expression and purification
[0030] Competent cells of Escherichia coli BL21 (DE3) were prepared by methods known in the art, and the wild-type expression vectors and mutant expression vectors constructed in Example 1 and Example 2 were transformed into competent cells by heat shock method, respectively. After cloning PCR and sequencing verification, it is ready for use.
[0031] The positive bacteria were picked by the inoculation needle and inoculated into 5mL LB medium, cultured at 30°C and 200r / min for 12h, and then inoculated into 400mL LB containing 50ug / mL kanamycin at a 2% (V / V) inoculation amount. medium, cultured at 30°C, 200 r / min for 8 h. When the bacterial density OD600 reached 0.7, 0.5 mM IPTG was added to induce expression; the cells were incubated overnight at 16°C with shaking at 170 rpm.
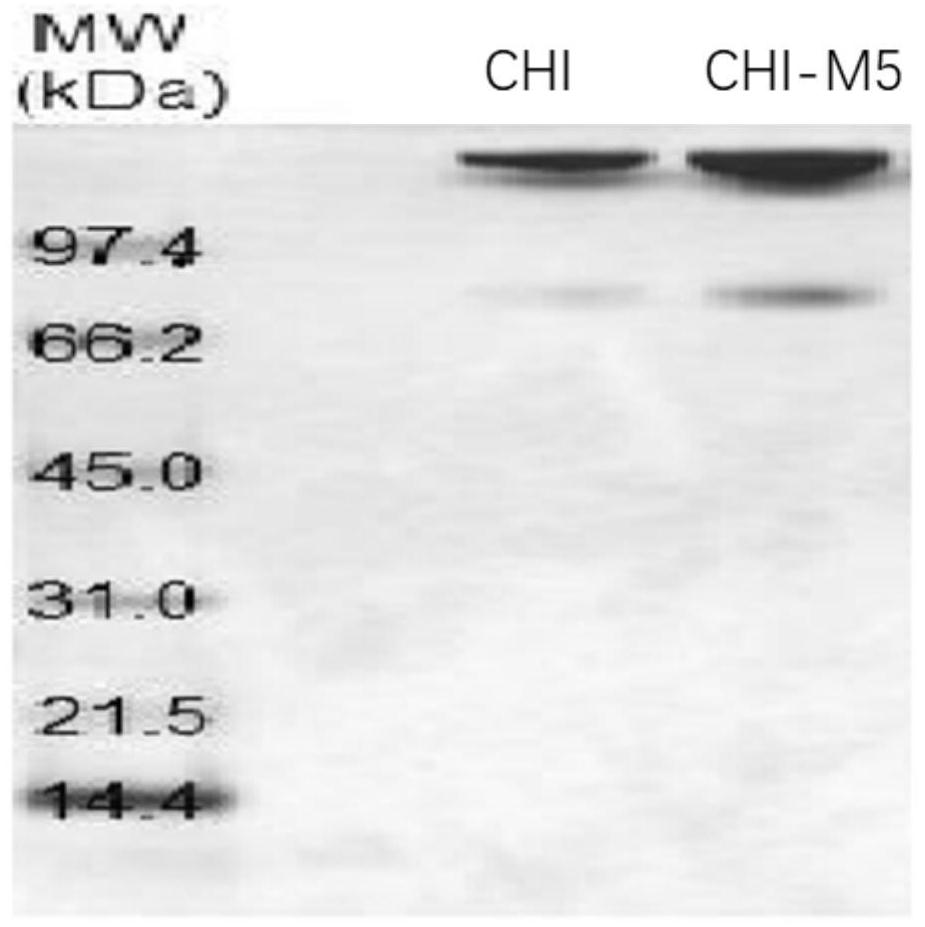
[0032] The bacteria were collected by centrifugation, washed with PBS, sonicated in ice, and the supernatant was collected by centrifugation. The AKTA a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com