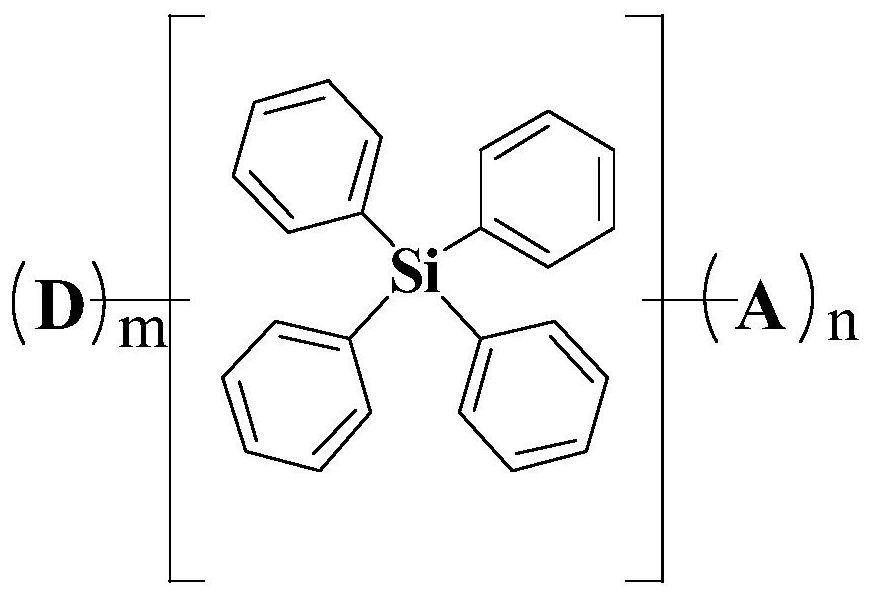
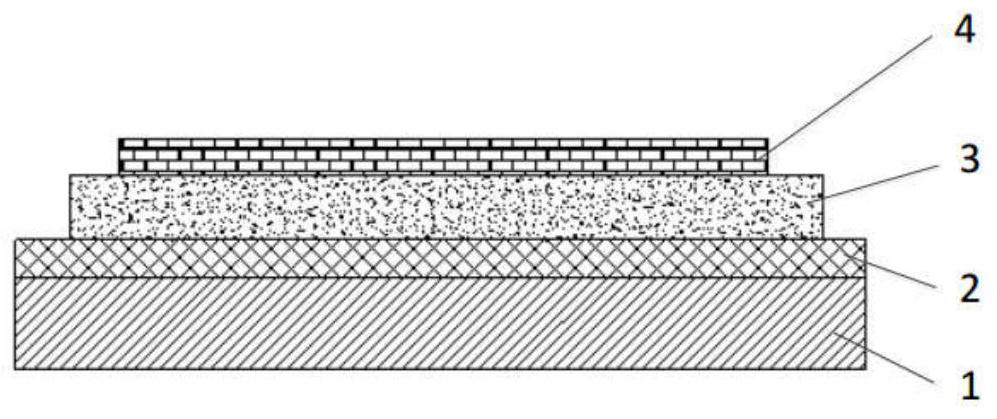
Compound, organic light emitting display panel and display device
A light-emitting display and compound technology, applied in silicon organic compounds, organic chemistry, light-emitting materials, etc., can solve the problems of poor stability of phosphorescent devices and roll-off of phosphorescent material efficiency, and achieve the suppression of non-radiative transition process and high-efficiency energy transfer , the effect of wide optical bandgap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Synthesis of Compound P1
[0086]
[0087] Weigh S1 (4.00mmol), S2 (2.00mmol), CuI (0.20mmol), trans-1,2-diaminocyclohexane (0.4mmol), anhydrous K 3 PO 4 (4.20 mmol), under a nitrogen atmosphere, 16 mL of toluene was added, and reacted at 110° C. for 24 h. The reaction mixture was cooled to room temperature. An appropriate amount of aqueous ammonium chloride solution was added to quench the reaction mixture. Extract the organic phase with dichloromethane (50mL) 3 times, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel column chromatography using n-hexane / dichloromethane (3 / 1) as eluent, and finally purified to give solid S3 (1.2 mmol, 60%).
[0088] MALDI-TOF MS: m / z calculated: C 36 h 26 BrNSi: 579.1; Measured: 579.4
[0089]
[0090] Under the condition of nitrogen protec...
Embodiment 2
[0103] Synthesis of Compound P8
[0104]
[0105] Weigh S1 (8.0mmol), S8 (4.0mmol), CuI (0.4mmol), trans 1,2-diaminocyclohexane (0.48mmol), anhydrous K 3 PO 4 (8.0 mmol), under a nitrogen atmosphere, 40 mL of toluene was added, and reacted at 110° C. for 24 h. The reaction mixture was cooled to room temperature. An appropriate amount of aqueous ammonium chloride solution was added to quench the reaction mixture. Extract the organic phase with dichloromethane (80mL) 3 times, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel column chromatography using n-hexane / dichloromethane (3 / 1) as eluent, and finally purified to give solid S9 (2.4 mmol, 60%).
[0106] MALDI-TOF MS: m / z calculated: C 39 h 32 BrNSi(S9): 621.2; Measured: 621.3.
[0107]
[0108] In a 100ml three-necked flask, first...
Embodiment 3
[0114] Synthesis of Compound P14
[0115]
[0116] Weigh S1 (6.0mmol), S12 (3.0mmol), CuI (0.3mmol), trans-1,2-diaminocyclohexane (0.35mmol), anhydrous K 3 PO 4 (6.0 mmol), under a nitrogen atmosphere, 40 mL of toluene was added, and reacted at 110° C. for 24 h. The reaction mixture was cooled to room temperature. An appropriate amount of aqueous ammonium chloride solution was added to quench the reaction mixture. Extract the organic phase with dichloromethane (80mL) 3 times, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel column chromatography using n-hexane / dichloromethane (3 / 1) as eluent, and finally purified to give solid S13 (1.95 mmol, 65%). MALDI-TOF MS: m / z, Calculated: C 48 h 36 BrNSi 2 :761.2; measured value: 761.4.
[0117]
[0118] In a 100ml three-necked flask, S13 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com