Alkynyl modified deoxyadenosine phosphoramidite monomer and preparation method thereof
A technology of deoxyadenosine phosphoramidite and deoxyadenosine, which is applied in the field of medicine, can solve the problems of complex synthetic methods, restrictions on practical application, and many steps, and achieve easy-to-obtain raw materials, high yield, simple reaction and post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
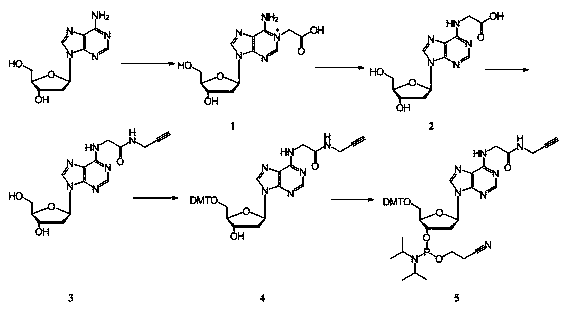
[0030] Example 1: Synthesis of the phosphoramidite monomer modified by the alkynyl group at the adenine base position
[0031] 1. Preparation of N1-carboxymethyl-deoxyadenosine (compound 1)
[0032] Pour 5ml of pH=7.0, 0.1mol / L phosphate buffer solution into a 10ml round bottom flask. Add 1500 mg of iodoacetic acid to the round bottom flask, then add 400 mg of deoxyadenosine, and dissolve with slight heat. Use 5mol / L NaOH solution to adjust the pH value to make pH=7.0. At this time, a large amount of white flocculent solids of inorganic salts were precipitated in the reaction solution, and the solids disappeared when the heating was continued to 50°C. The water bath was refluxed for reaction, the temperature was set to 50°C, and the reaction was carried out for 8 hours. After the reaction, the solution changed from orange to colorless and then to pink. Use 5 mol / L HCl to adjust the pH value of the solution to pH=3. The reaction solution was added to a precooled (-5°C) ace...
Embodiment 2
[0041] Synthesis of Example 2 Alkyne Modified DNA
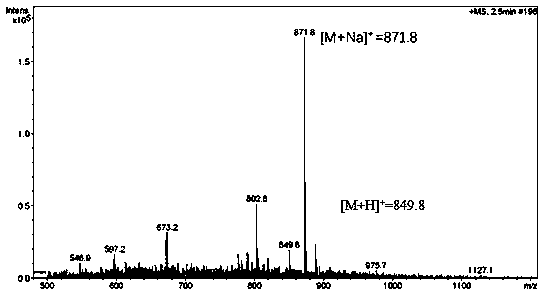
[0042] In the present invention, the alkynyl-modified deoxyadenosine phosphoramidite monomer compound is synthesized by a DNA synthesizer and purified by a high-performance liquid chromatography to obtain an alkynyl-modified oligonucleotide DNA. The product was confirmed by mass spectrometry, as Figure 4 shown.
[0043] The oligonucleotide sequences used in the present invention are as follows:
[0044] DNA: 5'-AAAA * AAAAAAAAAAAAATT-3'
[0045] where A * Indicates the site of alkynyl modification of deoxyadenosine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com