A kind of nitrendipine dispersible tablet and preparation method thereof
A technology of dispersible tablets and nitren, which is applied in the field of nitrendipine dispersible tablets and its preparation, and can solve the problems of low bioavailability, poor absorption, irregular blood pressure fluctuations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Nitrendipine Dispersible Tablets
[0051] Prescription composition:
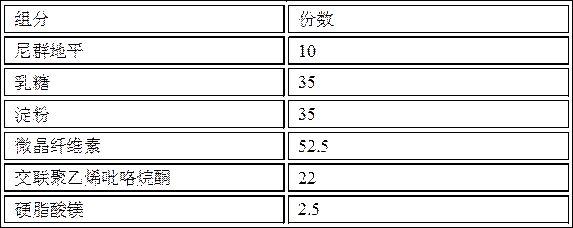
[0052] components weight (g) number of copies Nitrendipine 10 10 lactose 35 35 starch 35 35 microcrystalline cellulose 52.5 52.5 Cross-linked polyvinylpyrrolidone 22 22 Magnesium stearate 2.5 2.5
[0053] Preparation:
[0054] ① Micronization treatment of raw material medicine: micronization treatment of the raw material drug of nitrendipine to obtain more than 90% of the micropowder with a particle size of no more than 6 microns, and set aside;
[0055] ② Pretreatment of excipients: Microcrystalline cellulose, lactose, starch, cross-linked polyvinylpyrrolidone, and magnesium stearate are sieved through 120 sieves respectively, and set aside;
[0056] ③ Mixing of raw and auxiliary materials: using the method of equal increase, mix nitrendipine, microcrystalline cellulose, lactose, and cross-linked polyvinylpyrrolidone (3 / 5 of t...
Embodiment 2
[0062] Embodiment 2: Nitrendipine Dispersible Tablets
[0063] Prescription composition:
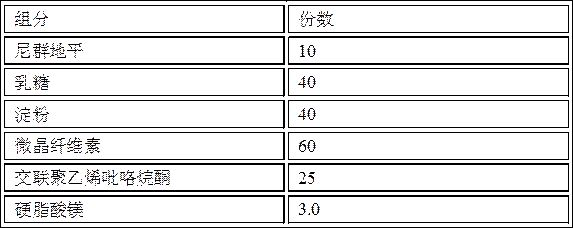
[0064] components weight (g) number of copies Nitrendipine 10 10 lactose 40 40 starch 40 40 microcrystalline cellulose 60 60 Cross-linked polyvinylpyrrolidone 25 25 Magnesium stearate 3 3
[0065] Preparation:
[0066] Same as Example 1
[0067] The nitrendipine dispersible tablet prepared in embodiment 2 is carried out disintegration time limit and dissolution rate inspection, and the results are as follows:
[0068] sample Disintegration time limit Dissolution Example 2 2.6 Compliance
Embodiment 3
[0069] Embodiment 3: Nitrendipine Dispersible Tablets
[0070] Prescription composition:
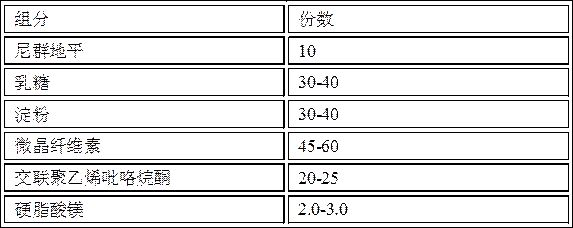
[0071] components weight (g) number of copies Nitrendipine 10 10 lactose 30 30 starch 30 30 microcrystalline cellulose 45 45 Cross-linked polyvinylpyrrolidone 20 20 Magnesium stearate 2 2
[0072] Preparation:
[0073] Same as Example 1
[0074] The nitrendipine dispersible tablet prepared by embodiment 3 is carried out to disintegration time limit and dissolution rate inspection, and the results are as follows:
[0075] sample Disintegration time limit Dissolution Example 3 2.4 Compliance
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com