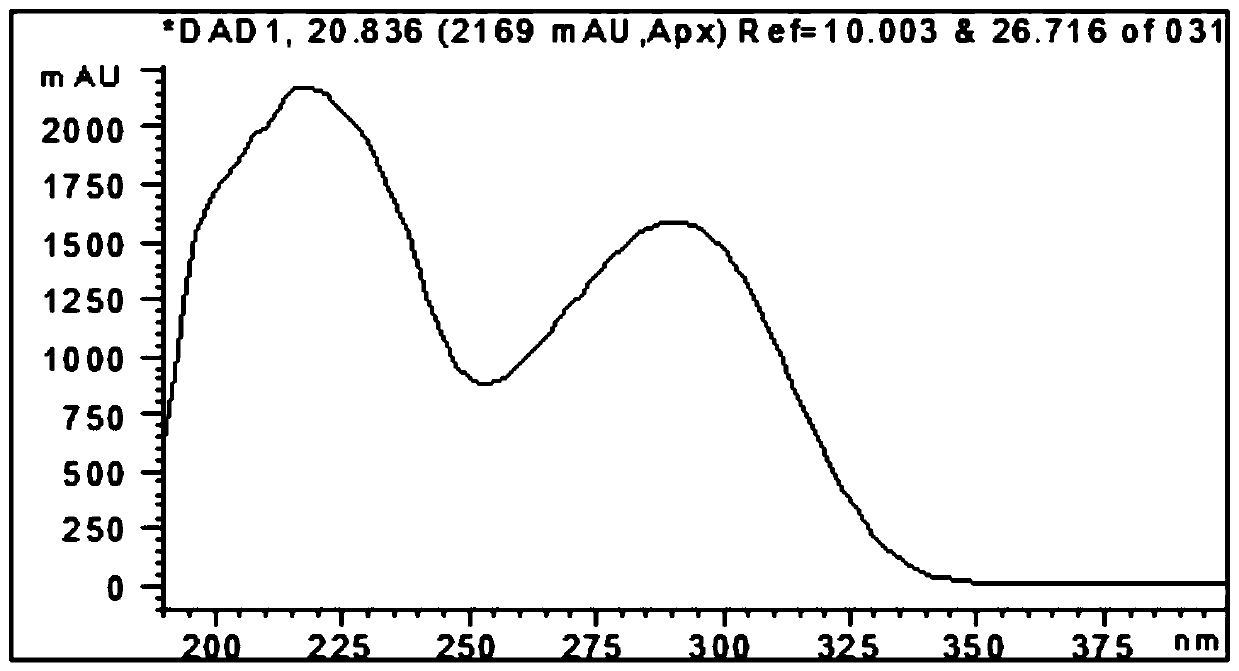
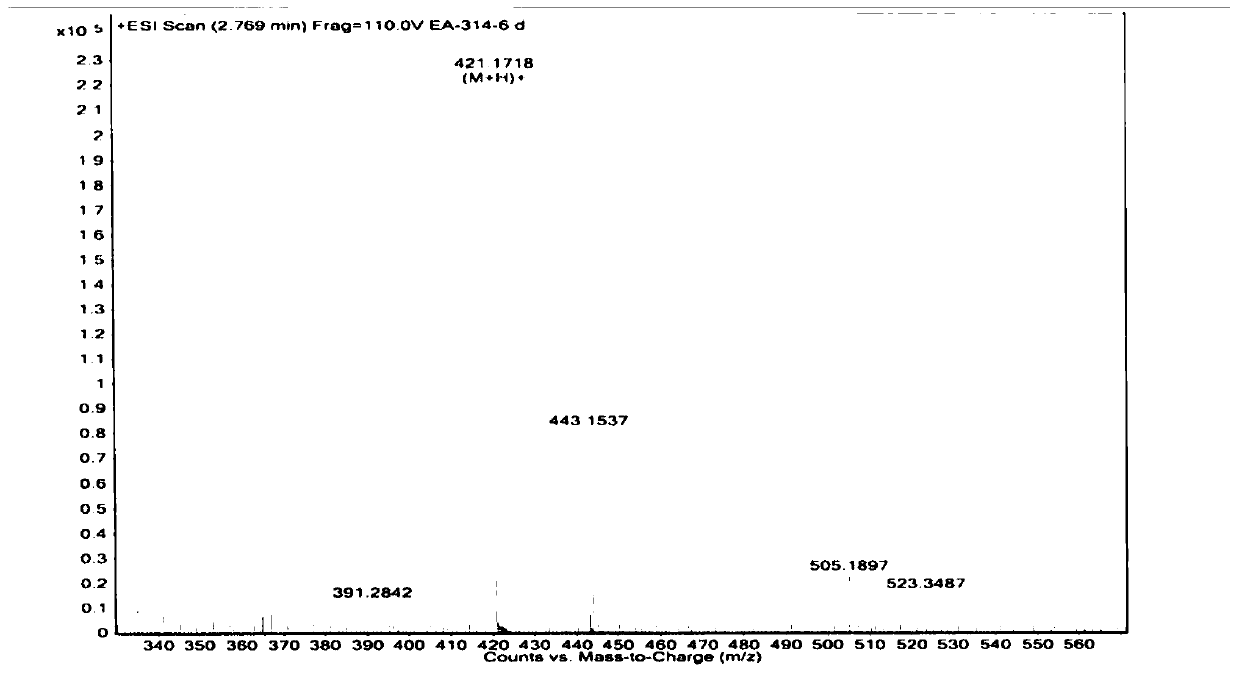
Compounds and application of compounds in preparation of antituberculosis drug
A compound and anti-tuberculosis technology, applied in the field of medicine, can solve problems such as the difficulty of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, application strain MS100137 production preparation compound
[0043] 1. Preparation of seed solution
[0044] 1. Spread MS100137 on a Ver01 solid plate and culture at 28°C for about 5 days until a single orange colony is formed.
[0045] The preparation method of plate medium: dissolve 10 grams of soluble starch, 10 grams of glucose, 10 grams of glycerin, 2.5 grams of corn steep liquor, 5 grams of peptone, 2 grams of yeast extract, 1 gram of sodium chloride, and 20 grams of agar powder in water, After adjusting the pH to 7.3, add 3 grams of calcium carbonate, dilute to 1 L, and sterilize at 115°C for 30 minutes.
[0046] 2. Divide the seed culture medium into 250 mL Erlenmeyer flasks (40 mL / bottle), pick a single colony of the bacterial species from the plate and inoculate it into the seed culture medium, shake and culture at 28° C. and 200 rpm for 5 days to obtain the seed liquid.
[0047] The preparation method of the seed medium: dissolve 10 grams of ...
Embodiment 2
[0079] Embodiment 2, anti-BCGMIC compound active test model
[0080] 7H9 medium (180ml): 0.94g 7H9 medium powder, 0.4mL glycerol, 0.1mL Tween80, sterilized at 121°C for 10min. Add 20 mL of filter-sterilized enrichment agent OADC just before use.
[0081] OADC (30 mL): NaCl 0.255g, BSA-V 1.5g, glucose 0.6g, sodium oleate 0.016g, catalase 0.0012g.
[0082] I.BCG cryopreservation tube live bacteria: Mycobacterium bovis Mycobacterium bovis attenuated strain bacillus Calmette-Guérin BCG (Pasteur 1137P2) is received in 10mL7H9 medium (adding filter-sterilized in the medium) OADC and 4 μL of 50 mg / mL kanamycin, the final concentration of kanamycin is 20 μg / ml), and cultured at 37°C and 110 rpm / min for 7-10 days (the culture time can be appropriately increased according to the growth of the bacterial solution).
[0083] II. Construction of MIC Compound Activity Test Model
[0084] The attenuated Mycobacterium bovis strain BCG was inoculated into a 250mL Erlenmeyer flask containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com