Aluminum alloy refining material and preparation method and application thereof
An aluminum alloy and composite technology, which is applied in the field of aluminum alloy refinement materials and their preparation, can solve the problems of difficult control of the preparation process, inability to undergo simultaneous metamorphic treatment, poor effect of modifiers, etc. Improve the efficiency of metamorphic treatment and save the effect of removing the slag step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
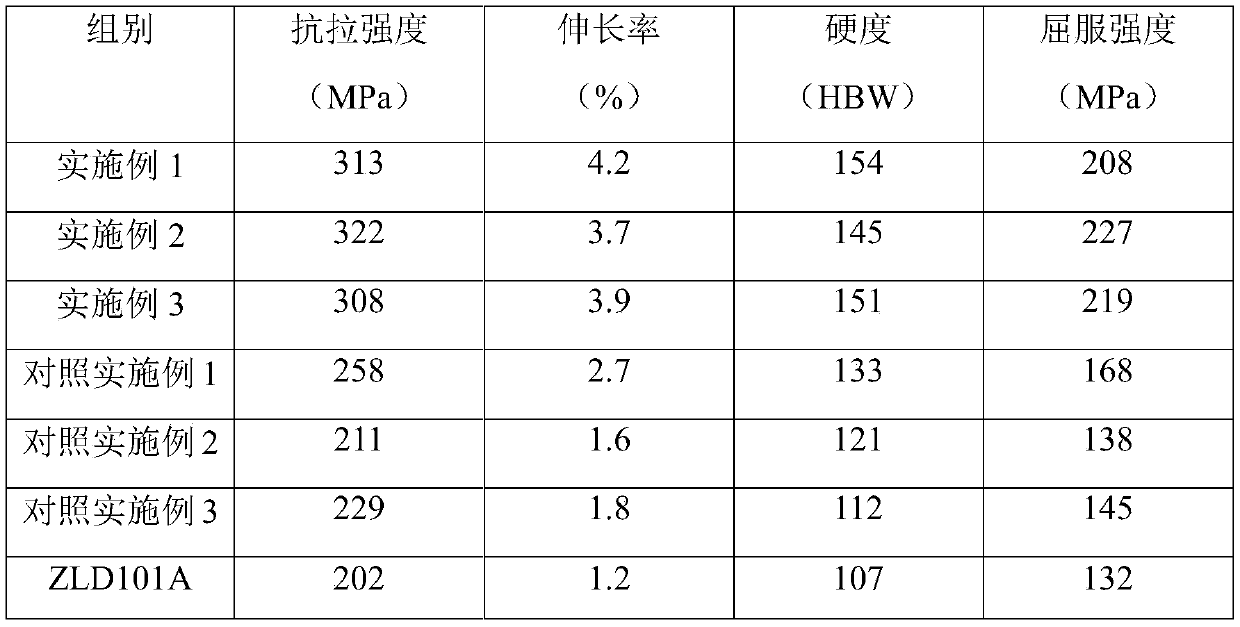
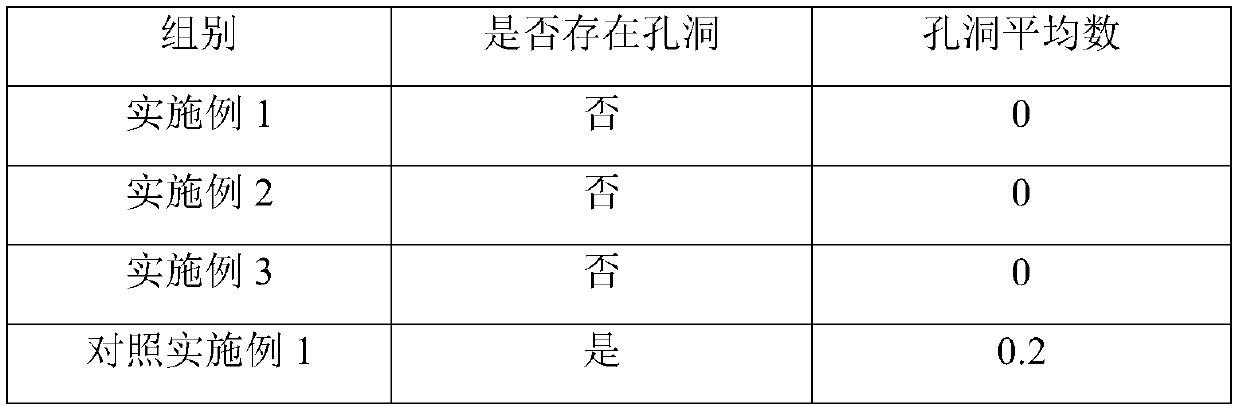
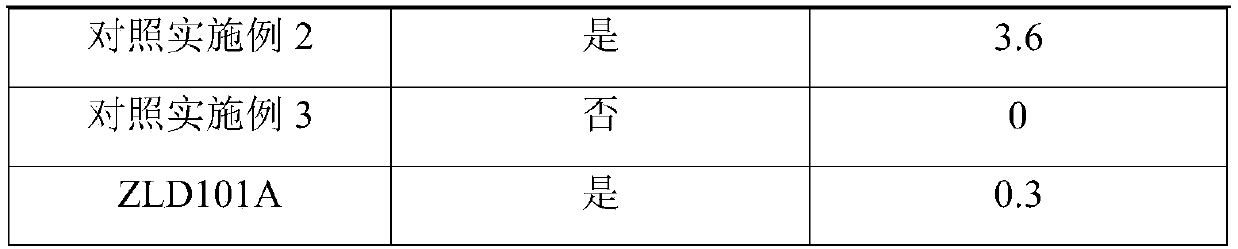
[0033] Embodiment 1: Aluminum alloy refinement materials were prepared as follows:
[0034] 1. Preparation of raw materials:
[0035] The composition of lepidolite powder is: Al 2 o 3 23.98wt%, H 2 O is 1.22wt%, FeO is 0.34wt%, MgO is 0.02wt%, CaO is 0.32wt%, TiO 2 ≤0.03wt%, K 2 O is 10.10wt%, Na 2 O is 0.05wt%, Li 2 O is 3.55wt%, MnO is 0.29wt%, F is 3.20wt%, Rb 2 O is 0.29wt%, Cs 2 O is 0.22wt%, the balance is SiO 2 , The particle size of lepidolite powder is 80 mesh.
[0036] Potassium fluoroaluminate, sodium fluoroaluminate, disodium hydrogen phosphate hydrate, potassium chloride, sodium chloride, barium acetate, barium nitrate, samarium fluoride, aluminum acetate, aluminum chloride, cryptocrystalline graphite, potassium carbonate, The purity of phosphotungstic acid, samarium nitrate and pure aluminum is greater than 99.9wt%, and the particle size is 100 mesh; the samarium nitrate is samarium nitrate hexahydrate;
[0037] ZLD101A aluminum alloy composition by we...
Embodiment 2
[0047] Embodiment 2: Aluminum alloy refined material is prepared as follows:
[0048] 1. Preparation of raw materials:
[0049] The composition of lepidolite powder is: Al2 o 3 28.34wt%, H 2 O is 2.09wt%, FeO is 1.08wt%, MgO is 0.09wt%, CaO is 1.22wt%, TiO 2 ≤0.03wt%, K 2 O is 12.45wt%, Na 2 O is 0.25wt%, Li 2 O is 4.07wt%, MnO is 0.89wt%, F is 4.90wt%, Rb 2 O is 0.42wt%, Cs 2 O is 0.63wt%, the balance is SiO 2 , The particle size of lepidolite powder is 100 mesh.
[0050] Potassium fluoroaluminate, sodium fluoroaluminate, disodium hydrogen phosphate hydrate, potassium chloride, sodium chloride, barium acetate, barium nitrate, samarium fluoride, aluminum acetate, aluminum chloride, cryptocrystalline graphite, potassium carbonate, The purity of phosphotungstic acid, samarium nitrate and pure aluminum is greater than 99.9wt%, and the particle size is 150 mesh; the samarium nitrate is samarium nitrate hexahydrate;
[0051] ZLD101A aluminum alloy composition by weight pe...
Embodiment 3
[0061] Embodiment 3: Aluminum alloy refinement materials were prepared as follows:
[0062] 1. Preparation of raw materials:
[0063] The composition of lepidolite powder is: Al 2 o 3 26.12wt%, H 2 O is 1.69wt%, FeO is 0.75wt%, MgO is 0.05wt%, CaO is 0.81wt%, TiO 2 ≤0.03wt%, K 2 O is 11.33wt%, Na 2 O is 0.14wt%, Li 2 O is 3.84wt%, MnO is 0.57wt%, F is 4.11wt%, Rb 2 O is 0.35wt%, Cs 2 O is 0.41wt%, the balance is SiO 2 , The particle size of lepidolite powder is 90 mesh.
[0064] Potassium fluoroaluminate, sodium fluoroaluminate, disodium hydrogen phosphate hydrate, potassium chloride, sodium chloride, barium acetate, barium nitrate, samarium fluoride, aluminum acetate, aluminum chloride, cryptocrystalline graphite, potassium carbonate, The purity of phosphotungstic acid, samarium nitrate and pure aluminum is greater than 99.9wt%, and the particle size is 130 mesh; the samarium nitrate is samarium nitrate hexahydrate;
[0065] ZLD101A aluminum alloy composition by we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com