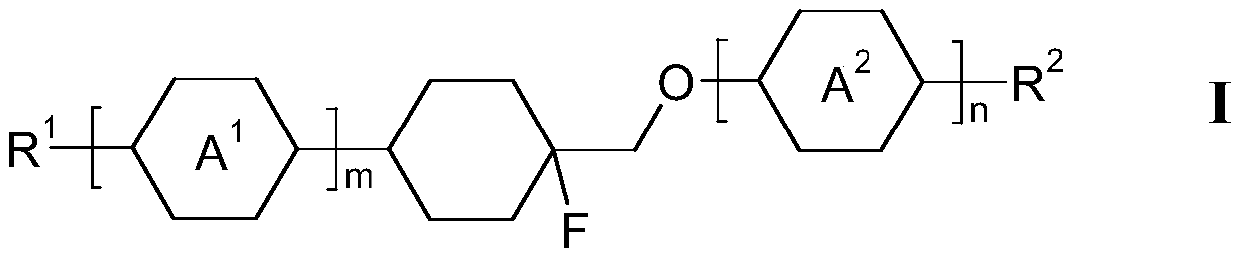
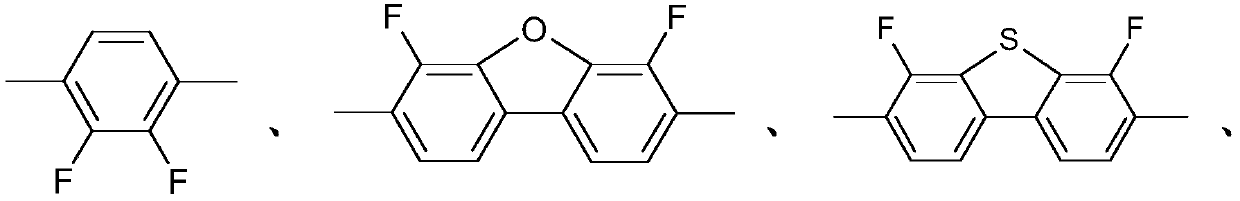
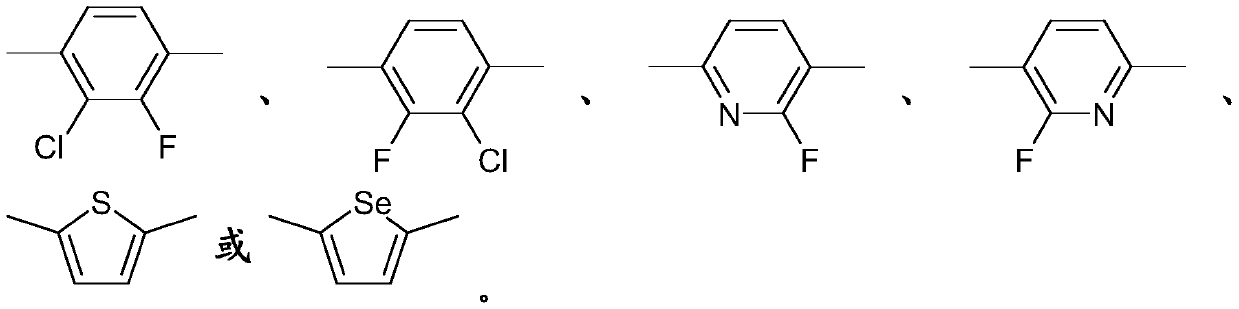
Monofluorinated cyclohexanes
A cyclohexenylene and alkyl group technology is applied in the field of electro-optical display elements to achieve the effect of good nematic phase width, rich mixture components and high-definition bright spots
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] step 1
[0117]
[0118] A solution of potassium tert-butoxide (21.9 g, 195.6 mmol) in dimethylsulfoxide (90 mL) was added dropwise to ketone 9 (29.0 g, 130.4 mmol) and trimethylsulfoxide iodide at room temperature (43.0 g, 195.6 mmol) in a mixture of dimethylsulfoxide (210 mL). The resulting mixture was stirred for 36 hours, then heptane (500 mL) was added and the mixture was stirred for a further 0.5 hours. The heptane phase was separated, and the Filtered, washed with water, washed with Na 2 SO 4 Dry and concentrate by evaporation in vacuo. The residue (16.7 g) was assisted with Puriflash (300 g SiO 2 , heptane / methyl tert-butyl ether 9:1) for purification. Epoxide 10 was obtained as colorless crystals (95.8% HPLC).
[0119] step 2
[0120]
[0121] A solution of HF in pyridine (65%, 5.0 mL, 185.5 mmol) was added dropwise to epoxide 10 (15.6 g, 61.8 mmol, HPLC: 93.7%) in dichloromethane (275 ml) in the solution. The resulting mixture was stirred at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com