A method for synthesizing fluorenone compounds by molecular oxygen oxidation in aqueous phase
A fluorene compound and a technology for synthesizing fluorenone, which are applied in the field of fluorenone compound synthesis, can solve problems such as organic solvent pollution, generation of by-products and the like, and achieve the effects of mild oxidation characteristics, easy separation, simple and safe post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation method of the fluorenone compound of following structural formula:
[0048]
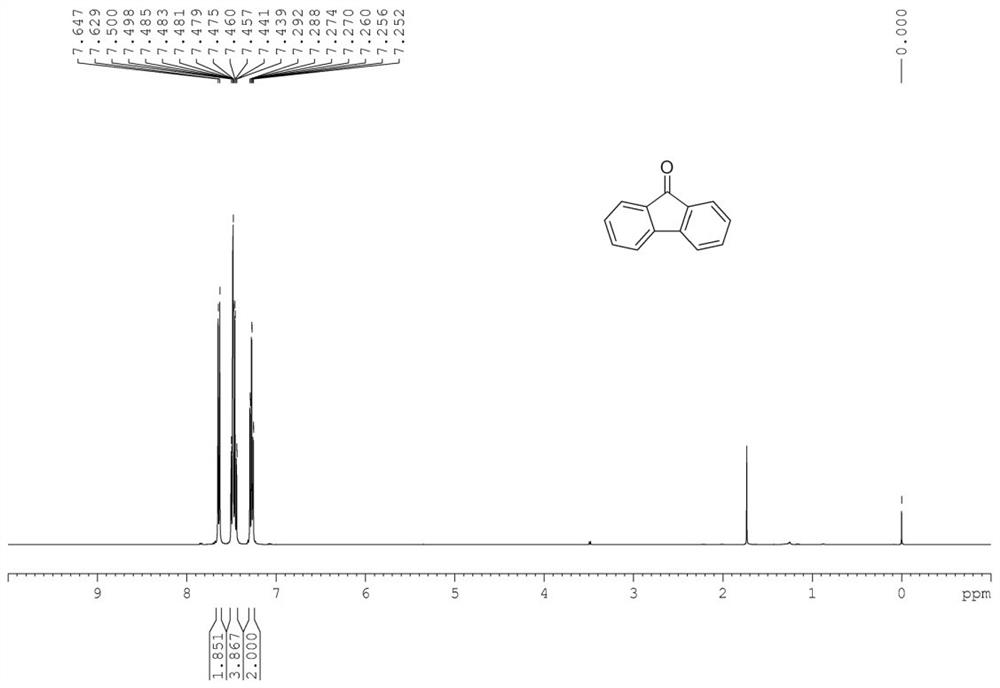
[0049] In the 50mL Shrek tube, add substrate fluorene 166mg (1mmol), the amount of copper chloride dihydrate added is based on the molar weight of Cu, and the addition of copper chloride dihydrate is 5% of the substrate molar weight, specifically 8.5mg, add the molar amount of ligand L1 is 5% of the substrate molar amount, specifically 25mg, add 40mg (1eq.) of sodium hydroxide and 5mL of water, add magnetons, and place in an oil bath at 40°C under air conditions Stir the reaction for 4 hours, and filter the reaction solution after the reaction to obtain 175 mg of the target product with a yield of 97%. Target product characterization data: Yellow solid.(PE / DCM=10:1as eluent).mp 79-81℃. 1 H NMR (400MHz, CDCl 3 )δ=7.64(d, J=7.3Hz, 2H), 7.51-7.43(m, 4H), 7.30-7.24(m, 2H). 13 C NMR (100MHz, CDCl 3 )δ=193.93, 144.43, 134.69, 134.15, 129.08, 124.30, 120.32. LRMS (EI): m / z calcd ...
Embodiment 2
[0051] The preparation method of the fluorenone compound of following structural formula:
[0052]
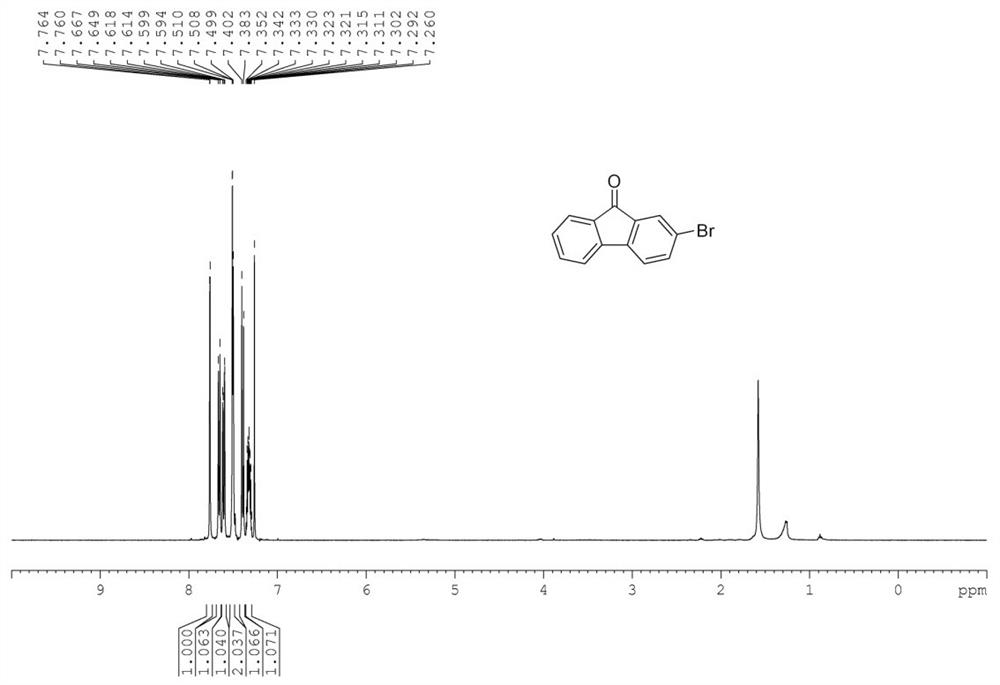
[0053] Add substrate 2-bromofluorene 244mg (1mmol) in the 50mL Shrek tube, the amount of ferric trichloride added is based on the molar weight of Fe, and the added amount of ferric trichloride is 10% of the substrate molar weight, specifically 3.2 mg, the molar amount of ligand L2 added is 10% of the substrate molar amount, specifically 5 mg, 168 mg (3 eq.) of potassium hydroxide and 1.8 mL of water were added, magnetons were added, oxygen was replaced by 0.1 MPa, the tube was sealed, 80 Stir and react in an oil bath at °C for 12 hours. After the reaction, the reaction solution is filtered and separated to obtain 232.5 mg of the target product with a yield of 90%. Yellow solid.(PE / DCM=10:1as eluent).mp 143-146℃. 1 H NMR (400MHz, CDCl 3 )δ=7.76(d,J=1.7Hz,1H),7.66(d,J=7.4Hz,1H),7.63-7.59(m,1H),7.52-7.48(m,2H),7.39(d,J =7.9Hz,1H),7.36-7.29(m,1H). 13 C NMR (100MHz, CDCl 3 )...
Embodiment 3
[0055] The preparation method of the fluorenone compound of following structural formula:
[0056]
[0057] Add substrate 2,7-dibromofluorene 320mg (1mmol) in 50mL Shrek tube, the amount of adding palladium acetate is based on the molar weight of Pb, the adding amount of palladium acetate is 0.05% of the substrate molar weight, specifically 0.12 mg, the molar amount of added ligand L1 is 0.05% of the substrate molar weight, specifically 0.6mg, add 3.26g (10eq.) of cesium carbonate and 18mL of water, add magnetons, replace oxygen with 0.5MPa, and place in an oil bath at 100°C The reaction was stirred for 16 hours. After the reaction, the reaction solution was filtered and separated to obtain 330 mg of the target product with a yield of 98%. Target product characterization data Yellow solid.(PE / DCM=10:1as eluent).mp203-205℃. 1 H NMR (400MHz, CDCl 3 )δ=7.68(d,J=1.8Hz,2H),7.57-7.52(m,2H),7.30(d,J=7.9Hz,2H). 13 C NMR (100MHz, CDCl 3 )δ=189.93, 141.23, 136.45, 134.23, 126.82,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com