A water-soluble transition metal complex catalyzed a method for synthesizing asymmetric sulfides in aqueous phase by molecular oxygen oxidation
A technology for the oxidation of water and transition metals with molecular oxygen, which is applied in the preparation of sulfides, organic chemistry, etc., can solve the problems of many reaction by-products, organic solvent pollution, etc., and achieves low by-products, mild oxidation characteristics, and high product selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The following structural formula has the preparation method of asymmetric sulfide:
[0051]
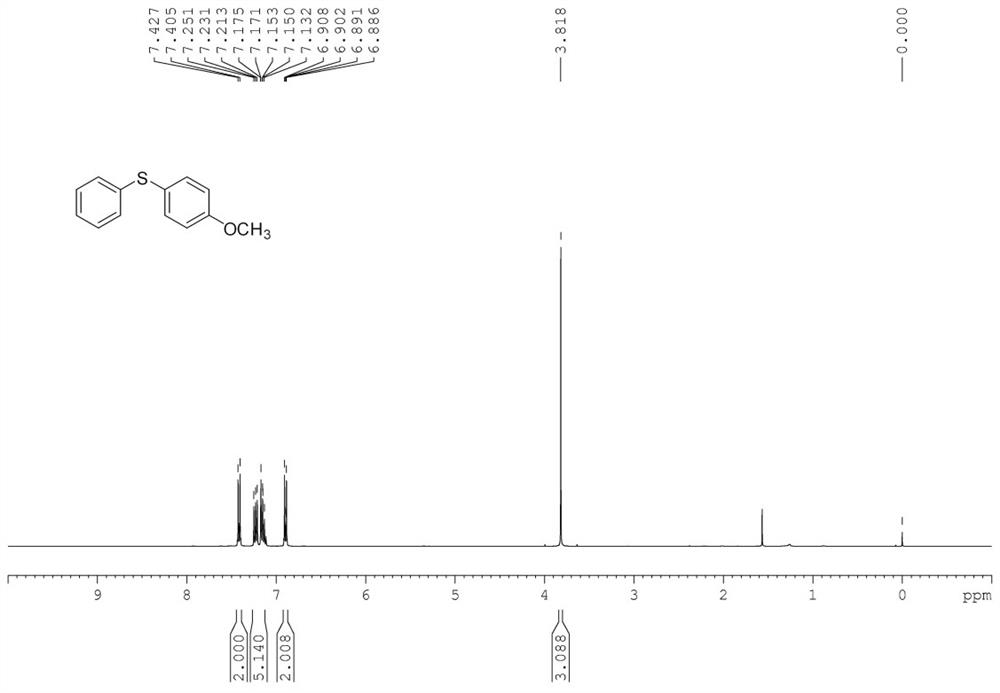
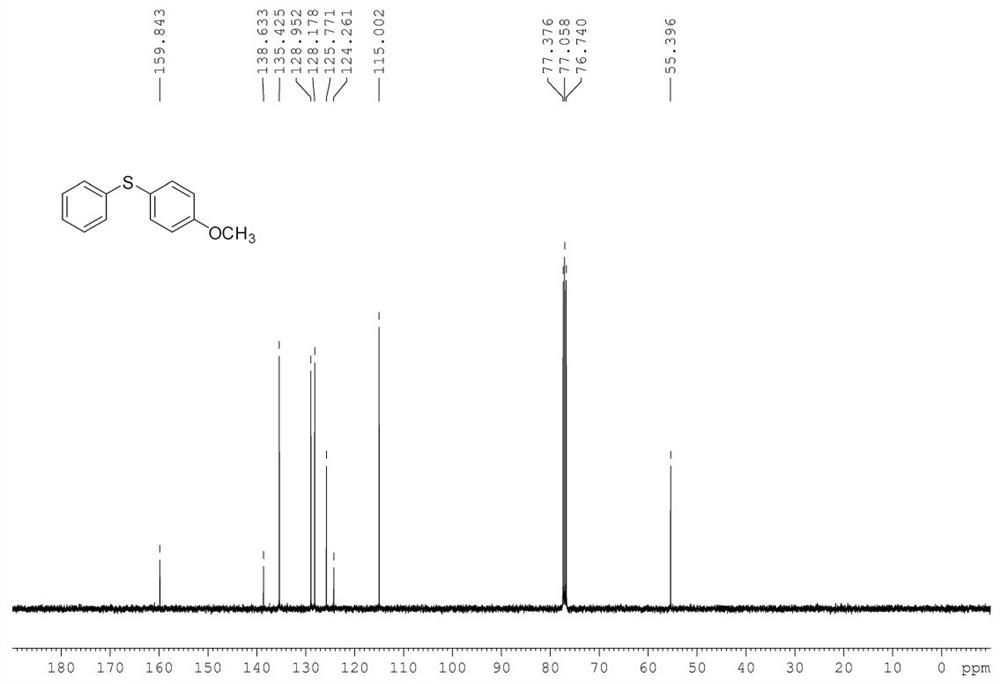
[0052] In the 50mL Shrek tube, add substrate phenylhydrazine 0.2mmol (22mg), p-methoxythiophenol 0.2mmol (28mg), copper acetate trihydrate 2.4mg (5mol%, the molar percentage is represented by the molar weight of metal ion The percentage of substrate molar weight is calculated, and the calculation method of other embodiments is the same), ligand L1, 5mg (5mol%), cesium carbonate 65mg (1eq.), water 2mL, add magneton, replace oxygen 0.1MPa, Stir the reaction in an oil bath at 100°C for 12h. After the reaction, the reaction tube was cooled to room temperature, 50 mL of saturated brine was added, extracted three times with dichloromethane (3*50 mL), dried by adding anhydrous sodium sulfate for 30 min, and then the low boiling point solvent was removed by a rotary evaporator. Then, the reaction mixture was separated and purified by column chromatography (silica gel column, 30*300...
Embodiment 2
[0054] The following structural formula has the preparation method of asymmetric sulfide:
[0055]
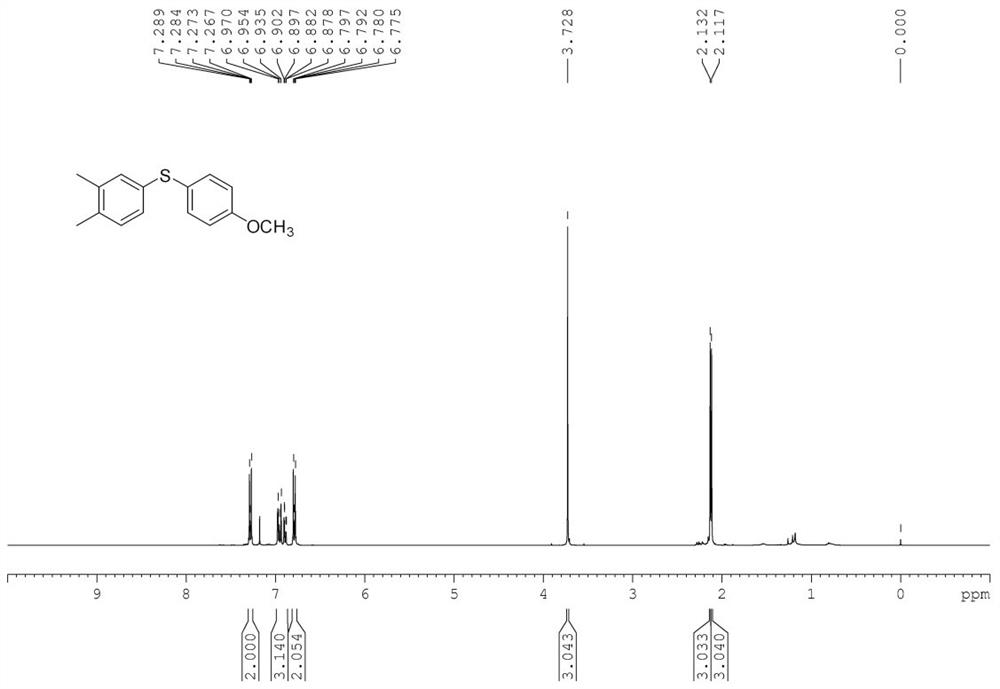
[0056] Add substrate 3,4-dimethylphenylhydrazine 0.2mmol (27mg), p-methoxythiophenol 0.2mmol (28mg), copper acetate trihydrate 4.8mg (10mol%), ligand L2, 10mg (10mol%), cesium carbonate 650mg (10eq.), water 2mL, add magneton, replace oxygen 0.3MPa, stir and react in 40°C oil bath for 5h. After the reaction, the reaction tube was cooled to room temperature, 50 mL of saturated brine was added, extracted three times with dichloromethane (3*50 mL), dried by adding anhydrous sodium sulfate for 30 min, and then the low boiling point solvent was removed by a rotary evaporator. Then, the reaction mixture was separated and purified by column chromatography (silica gel column, 30*300mm) (eluent: n-hexane:dichloromethane=2:1) to obtain the target product with a yield of 92%. Target product characterization data: Yellow oil.(hexane:DCM=2:1as eluent). 1 H NMR (400MHz, CDCl 3 )δ=7.31...
Embodiment 3
[0058] The following structural formula has the preparation method of asymmetric sulfide:
[0059]
[0060] Add substrate 3-chlorophenylhydrazine 0.2mmol (28mg), p-methoxythiophenol 0.2mmol (28mg), copper chloride dihydrate 0.03mg (0.05mol%), ligand L2, 0.05mg (0.05mol%), potassium carbonate 84mg (3eq.), water 2mL, add magneton, replace oxygen 0.2MPa, stir and react in 80°C oil bath for 10h. After the reaction, the reaction tube was cooled to room temperature, 50 mL of saturated brine was added, extracted three times with dichloromethane (3*50 mL), dried by adding anhydrous sodium sulfate for 30 min, and then the low boiling point solvent was removed by a rotary evaporator. Then, the reaction mixture was separated and purified by column chromatography (silica gel column, 30*300mm) (eluent: n-hexane:dichloromethane=2:1) to obtain the target product with a yield of 98%. Target product characterization data: Yellow solid.(hexane:DCM=2:1as eluent).50-53℃. 1 H NMR (400MHz, C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com