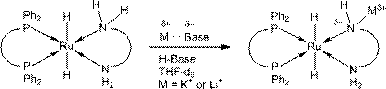Metal complex catalyst, and preparation method and application thereof
A technology of metal complexes and preparation methods, which can be applied in the directions of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, catalytic reactions, etc., can solve the problems of increased production cost, difficult separation, etc., and achieve excellent catalysis The effect of hydrogenation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
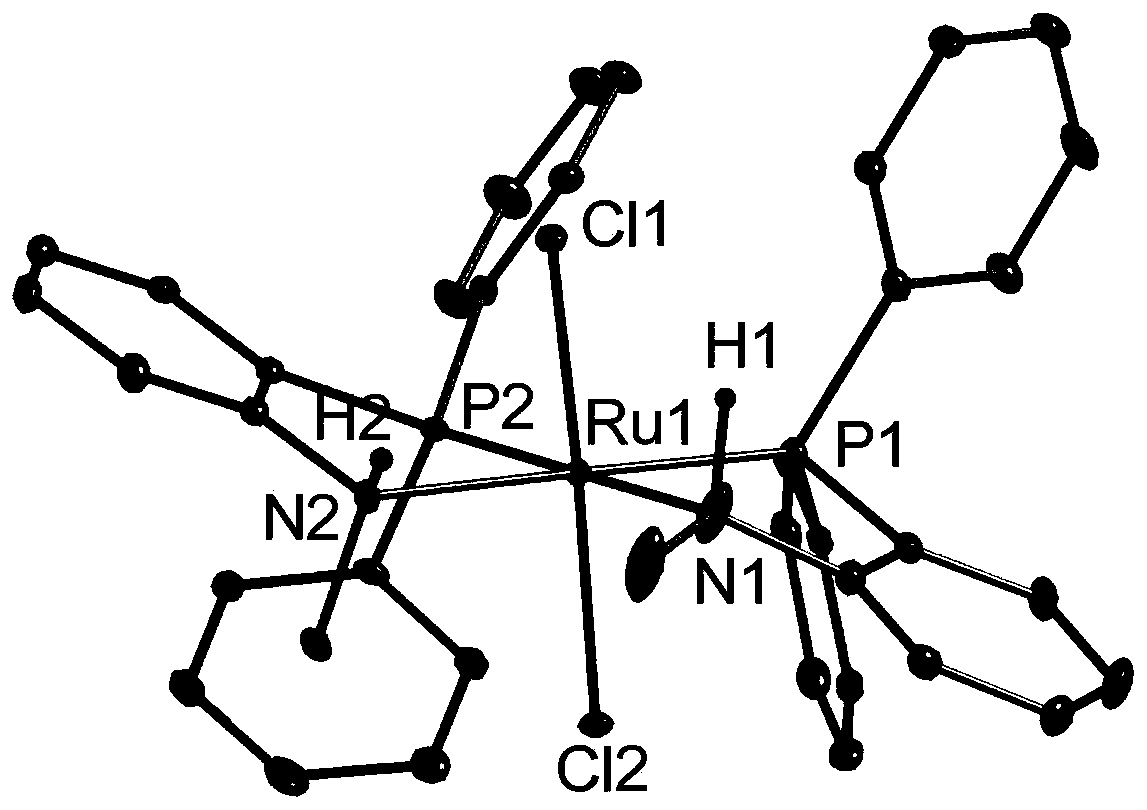
[0036] metal complex catalyst (o-PPh 2 C 6 h 4 NH 2 ) 2 RuCl 2 Preparation of (I-A-1):
[0037] Step (1): under an inert atmosphere, weigh 0.48g (PPh 3 ) 3 RuCl 2 (0.5mmol) and 0.14g o-Ph 2 PC 6 h 4 NH 2 (0.5 mmol) in a Schlenk bottle (100 mL) containing about 40 mL of toluene, and heated to 70° C. in a sealed manner. After reacting overnight, the reaction solution was cooled to room temperature, collected by filtration and washed with n-hexane to generate precipitates, dried under reduced pressure, weighed 0.31g (yield 87%), and obtained intermediate i[(PPh 3 )(o-Ph 2 PC 6 h 4 NH 2 )RuCl 2 ] 2 ;
[0038] Step (2): Under an inert atmosphere, weigh 0.29g of intermediate i (0.2mmol) obtained in step 1 and 0.11g of o-Ph 2 PC 6 h 4 NH 2 (0.4 mmol) in a Schlenk bottle (100 mL) containing about 30 mL of toluene, and heated to 100°C in a sealed manner. After one day of reaction, the resulting yellow-green solid precipitate was collected by filtration and washe...
Embodiment 2
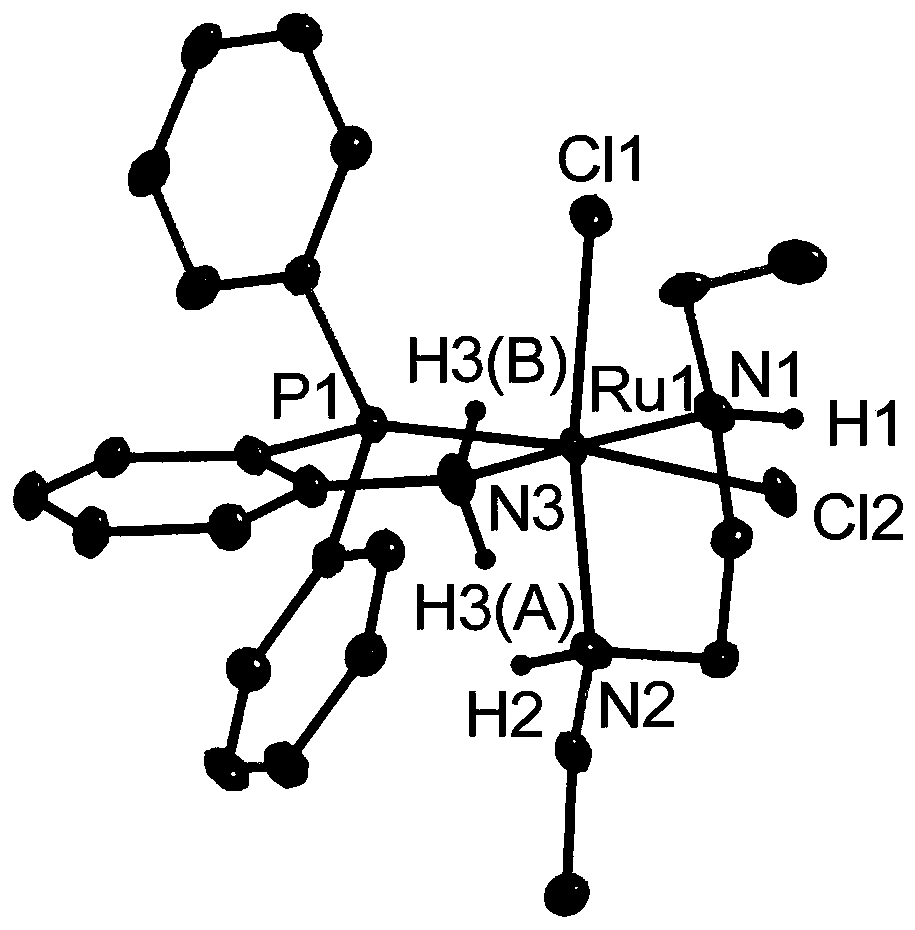
[0043] metal complex catalyst (o-PPh 2 C 6 h 4 NHMe) 2 RuCl 2 Preparation of (I-A-2):
[0044] Step (1): Under an inert atmosphere, weigh 0.48g RuCl 2 (DMSO) 4 (1.0mmol) and 0.29g o-Ph 2 PC 6 h 4 NHMe (1.0 mmol) was placed in a Schlenk bottle (100 mL) containing about 40 mL of tetrahydrofuran, and heated to 70°C in a sealed manner. After reacting overnight, the resulting precipitate was collected by filtration and washed with n-hexane, dried under reduced pressure, and weighed 0.40 g (yield 64%) to obtain the intermediate i(o-Ph 2 PC 6 h 4 NHMe)RuCl 2 (DMSO) 2 ;
[0045] Step (2): Under an inert atmosphere, weigh 0.18g of intermediate i (0.3mmol) obtained in step 1 and 87.0mg of o-Ph 2 PC 6 h 4 NHMe (0.3 mmol) was placed in a Schlenk bottle (100 mL) containing about 30 mL of toluene, and heated to 100°C in a sealed manner. After one day of reaction, the reaction solution was concentrated to about 1 mL and then about 5 mL of n-hexane was added. The orange prec...
Embodiment 3
[0050] metal complex catalyst (o-PPh 2 C 6 h 4 NHEt) 2 RuCl 2 Preparation of (I-A-3):
[0051] Step (1): Under an inert atmosphere, weigh 0.48g RuCl 2 (DMSO) 4 (1.0mmol) and 0.30g o-Ph 2 PC 6 h 4 NHEt (1.0 mmol) was heated to 70°C in a Schlenk bottle (100 mL) containing about 40 mL of tetrahydrofuran. After reacting overnight, the resulting precipitate was collected by filtration and washed with n-hexane, dried under reduced pressure, and weighed 0.44 g (yield 70%) to obtain intermediate i(o-Ph 2 PC 6 h 4 NHEt)RuCl 2 (DMSO) 2 ;
[0052] Step (2): Under an inert atmosphere, weigh 0.19g of intermediate i (0.3mmol) obtained in step 1 and 91.6mg of o-Ph 2 PC 6 h 4 NHEt (0.3 mmol) was heated to 100°C in a Schlenk bottle (100 mL) containing about 30 mL of toluene. After reacting for four days, the reaction solution was concentrated to about 1 mL and then 5 mL of n-hexane was added. The resulting pale orange precipitate was collected by filtration and washed with n-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com