Multi-iodine modified fluoroboron dipyrrole derivatives and its preparation method and application
A technology of pyrroles and derivatives, applied in the field of photosensitizers, can solve the problems of single photodynamic therapy effect of photosensitizers, limited application of low intersystem crossing efficiency, poor water solubility, etc., so as to improve photodynamic therapy activity and improve singlet state. Oxygen yield, water solubility improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
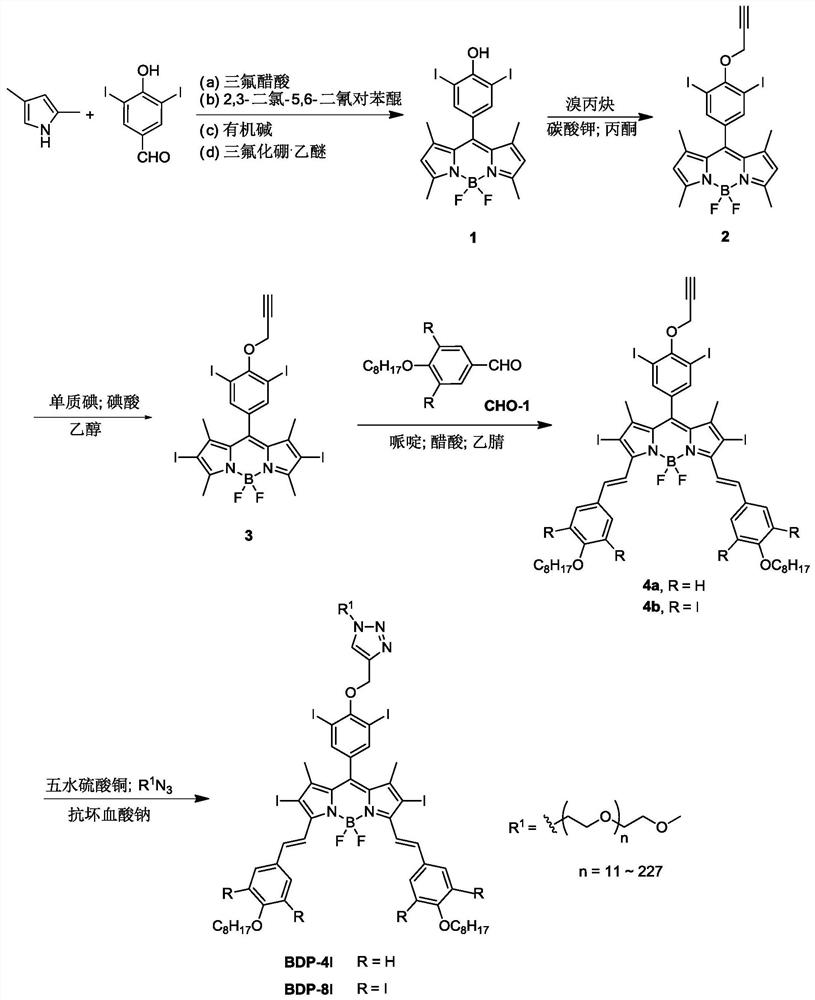
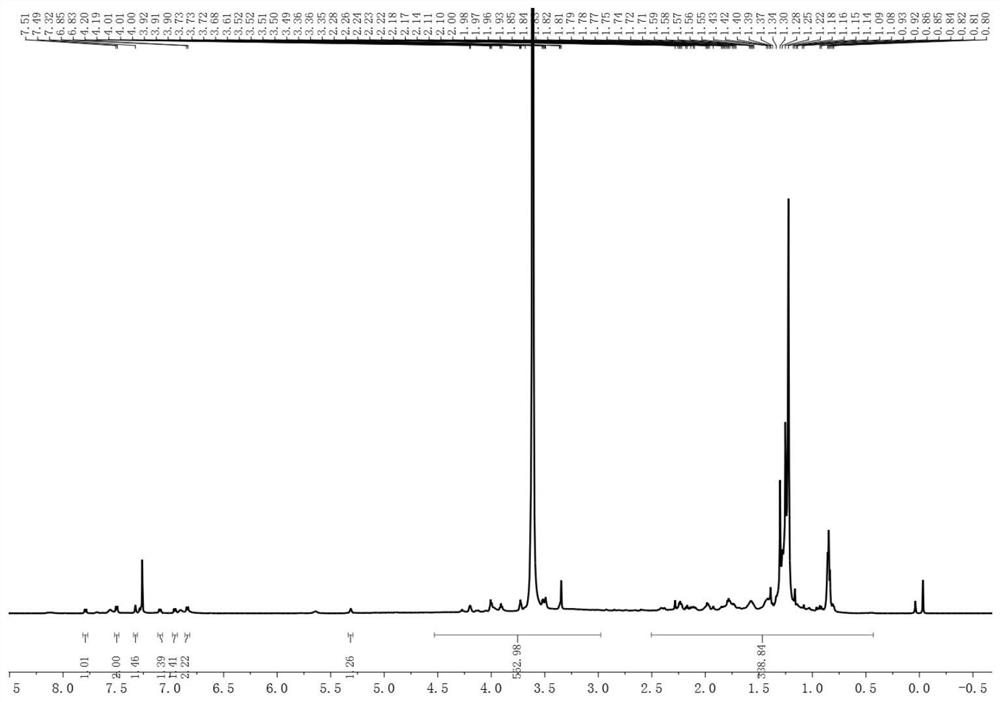
[0042] 1. Synthesis of Compound 1
[0043]Add 3,5-diiodo-p-hydroxybenzaldehyde and 2,4-dimethylpyrrole into the reaction vessel at a molar ratio of 1:2, and then add tetrahydrofuran 100 times the weight of 2,4-dimethylpyrrole as Solvent and 3-5 drops of trifluoroacetic acid as catalyst. Stir at 25°C for 12 hours. Subsequently, 2,3-dichloro-5,6-dicyano-p-benzoquinone dissolved in 2,4-bis In tetrahydrofuran with 10 times the weight of methylpyrrole, the reaction was stirred at room temperature for 12 hours. Finally, triethylamine 50 times the weight of 2,4-dimethylpyrrole was added, and boron trifluoride·ethyl ether 50 times the weight of 2,4-dimethylpyrrole was added dropwise in an ice-water bath to react overnight. After the reaction, filter with a sandboard Buchner funnel, concentrate under reduced pressure and rotate, add a small amount of dilute hydrochloric acid and stir for 3 hours. After concentrating again, it was extracted with dichloromethane. The extract was sub...
Embodiment 2
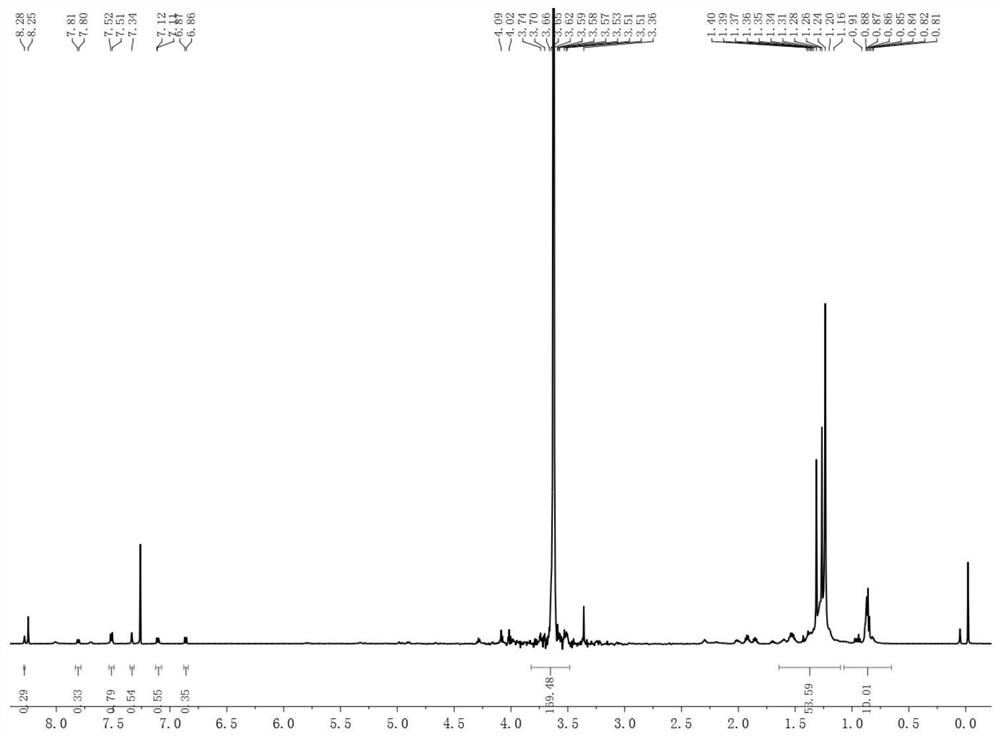
[0053] 1. Synthesis of Compound 1
[0054] Add 3,5-diiodo-p-hydroxybenzaldehyde and 2,4-dimethylpyrrole to the reaction vessel at a molar ratio of 1:3, and then add 50 times the weight of 2,4-dimethylpyrrole. Methane was used as solvent and 3-5 drops of trifluoroacetic acid as catalyst. Stir at 25°C for 24 hours. Subsequently, 2,3-dichloro-5,6-dicyano-p-benzoquinone dissolved in 2,4-bis 20 times the weight of methylpyrrole in tetrahydrofuran, stirred at room temperature for 12 hours. Finally, triethylamine 25 times the weight of 2,4-dimethylpyrrole was added, and boron trifluoride·ethyl ether 25 times the weight of 2,4-dimethylpyrrole was added dropwise in an ice-water bath to react overnight. After the reaction, filter with a sandboard Buchner funnel, concentrate under reduced pressure and rotate, add a small amount of dilute hydrochloric acid and stir for 1.5 hours. After concentrating again, it was extracted with dichloromethane. The extract was subjected to column chr...
Embodiment 3
[0064] 1. Synthesis of Compound 1
[0065] Add 3,5-diiodo-p-hydroxybenzaldehyde and 2,4-dimethylpyrrole to the reaction vessel at a molar ratio of 1:2, and then add 50 times the weight of 2,4-dimethylpyrrole Methane was used as solvent and 3-5 drops of trifluoroacetic acid as catalyst. Stir at 25°C for 24 hours. Subsequently, 2,3-dichloro-5,6-dicyano-p-benzoquinone dissolved in 2,4-bis 20 times the weight of methylpyrrole in tetrahydrofuran, stirred at room temperature for 12 hours. Finally, diisopropylamine 25 times the weight of 2,4-dimethylpyrrole was added, and boron trifluoride·ethyl ether 30 times the weight of 2,4-dimethylpyrrole was added dropwise in an ice-water bath to react overnight. After the reaction, filter with a sandboard Buchner funnel, concentrate under reduced pressure and rotate, add a small amount of dilute hydrochloric acid and stir for 6 hours. After concentrating again, it was extracted with dichloromethane. The extract was subjected to column chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com