Pentamethine-cyanine-dye photosensitive dye with long lifetime of excited state, and preparation method and application thereof
A photosensitive dye and excited state technology, applied in organic dyes, medical preparations containing active ingredients, azo dyes, etc., can solve the problems of fluorescence quenching, short life, shortened state life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthesis of embodiment 1. photosensitive molecule ANOMe-Cy5:
[0059]
[0060] (1) Synthesis of intermediate compound 3:
[0061] Compound 2 (0.34g, 1mmol), quaternary ammonium salt 1 (0.63g, 2mmol) and anhydrous sodium acetate (0.2g, 2.5mmol) in absolute ethanol were stirred under reflux for 2h. After cooling, the ethanol was spin-dried by a rotary evaporator, and the crude product was purified by silica gel column chromatography (dichloromethane / methanol=10:1, v / v) to obtain compound 3 as a blue powder (0.53 g, 71.8%). 1 HNMR (400MHz, MeOD): 8.41 (d, J = 13.3Hz, 2H, CH), 7.56 (d, J = 7.4Hz, 2H, ArH), 7.46 (t, J = 7.6Hz, 2H, ArH), 7.40 (d, J=7.8Hz, 2H, ArH), 7.33(t, J=7.4Hz, 2H, ArH), 6.49(d, J=13.3Hz, 2H, CH), 4.24(q, J=7.2Hz, 4H,CH 2 ),1.76(s,12H,CH 3 ), 1.45(t, J=7.2Hz, 6H, CH 3 ).ESI-MS (C 29 h 34 BrN 2 I) m / z: [M–I] - calcd.489.19; found, 489.28.
[0062] (2) Synthesis of photosensitive molecule ANOMe-Cy5:
[0063] Dissolve 3 (0.1g, 0.16mmol), ...
Embodiment 2
[0064] Embodiment 2. Singlet oxygen performance test of photosensitive molecule ANOMe-Cy5
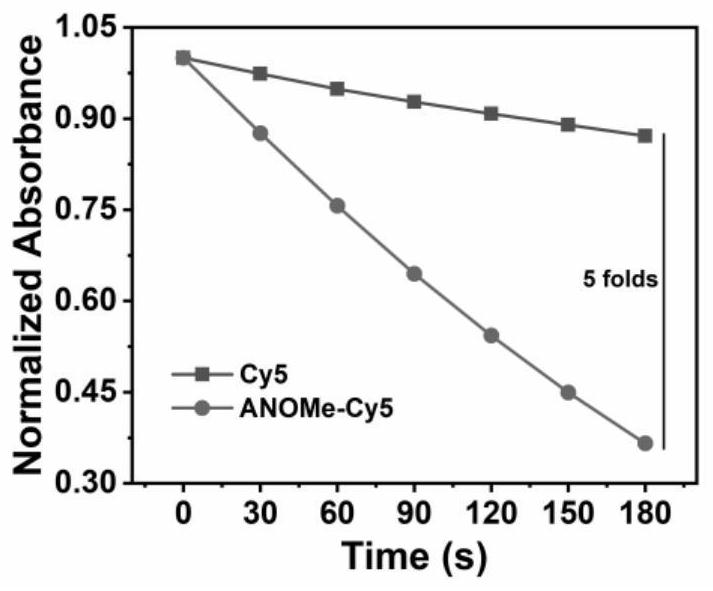
[0065] Add 1.5μM photosensitive molecule ANOMe-Cy5 to the test quartz dish containing 3mL dichloromethane solution, adjust the absorbance at 415nm to about 1 through the DPBF solution, place the dish at 660nm, 10mW / cm 2 Irradiate under a xenon light source, and record the absorption spectrum of the solution every 15 seconds. The test results are collated and displayed in figure 1 In , the absorption of the solution attenuates at a certain value as the irradiation time increases, indicating that the solution produces singlet oxygen under the irradiation of the light source of this wavelength.
Embodiment 3
[0066] The test of embodiment 3.ANOMe-Cy5 excited state lifetime
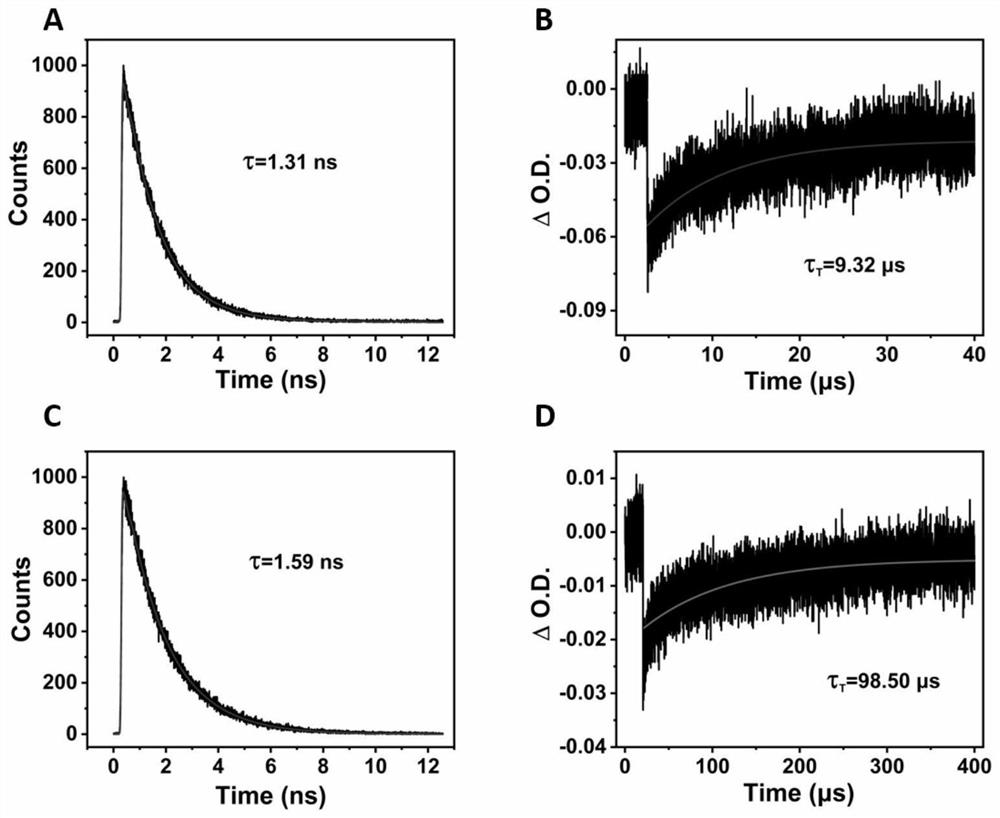
[0067] 5 μM of compound ANOMe-Cy5 was placed in 3 mL of dichloromethane solution. The fluorescence lifetime and triplet lifetime of the two compounds were tested by flash photolysis and time-resolved spectroscopy. The results are reflected in figure 2 Figures C and D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com