A kind of ligand compound based on bipyridine and its preparation method and application
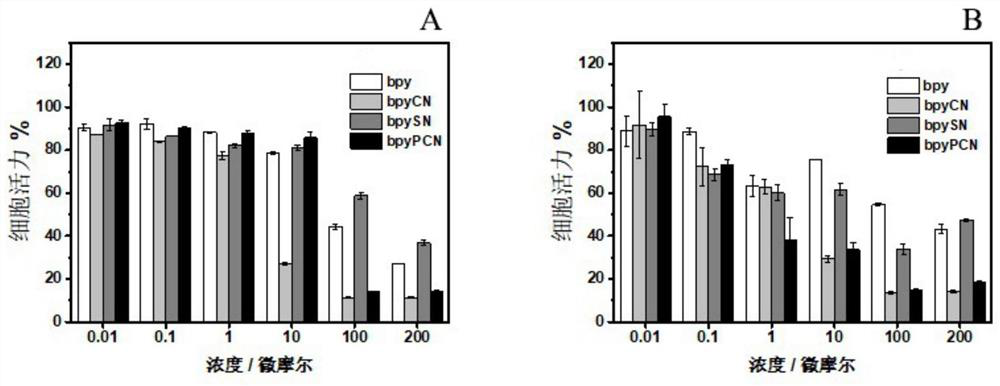
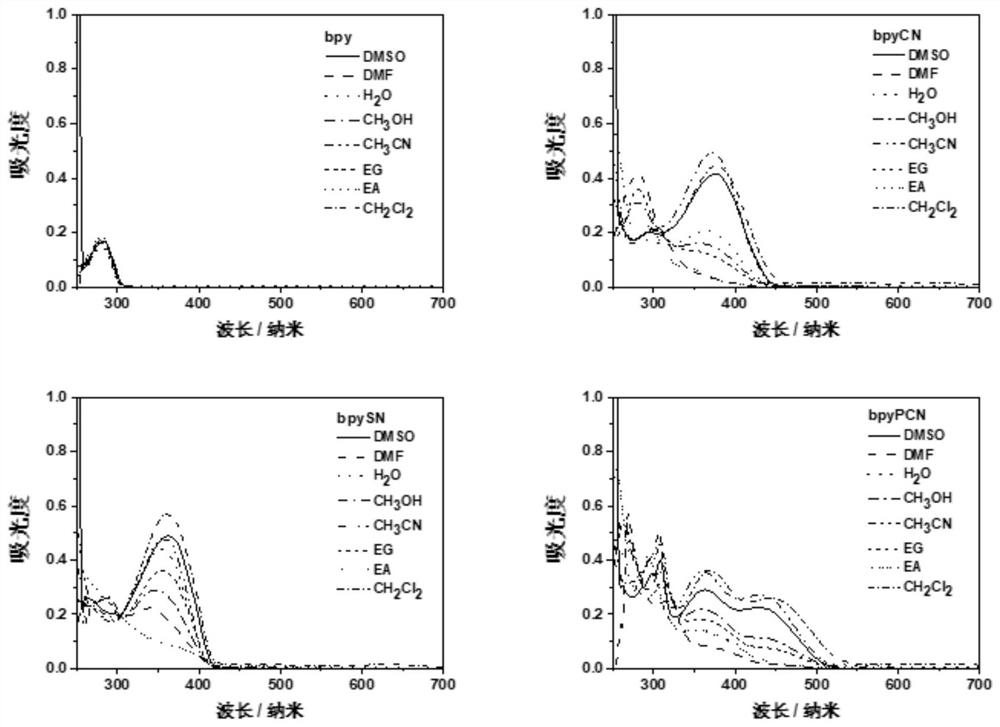
A ligand compound and bipyridine technology, applied in the field of pharmaceutical compounds, can solve the problems of poor light absorption performance and low photodynamic therapy activity, achieve good absorption capacity, improve photodynamic therapy activity, and strong growth inhibition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
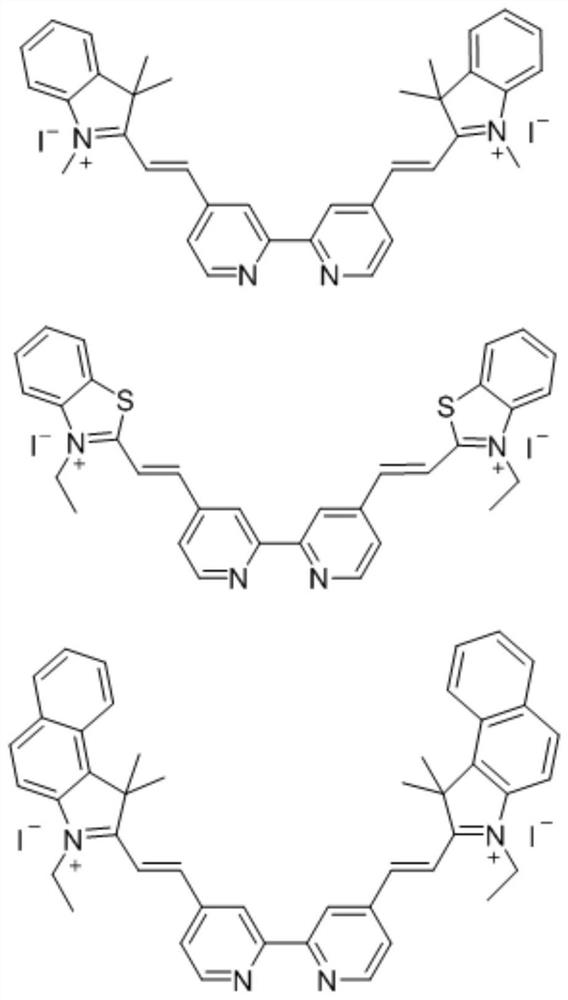
[0038] The synthesis of bpyCN compound, its structural formula is:
[0039]
[0040] The synthesis method of bpyCN is as follows:
[0041] Mixture of 2,2'-bipyridine-4,4'-dicarbaldehyde (0.106g, 0.5mmol) and 1,2,3,3-tetramethyl-3H-indolium iodide (0.301g, 1mmol) Heated to 80°C in pyridine / ethanol (16mL; 1:15v / v), reacted in the dark under an argon atmosphere for 19 hours, then cooled the reaction to room temperature, filtered and washed the precipitate with ether, and filtered the obtained crude product through ethanol Red-brown crystals were obtained by recrystallization, and the obtained crystals were further dried to obtain 0.241 g of red-brown powder with a yield of 62%. Characterized by NMR, its molecular formula is C 36 h 36 I 2 N 4 , 1 H NMR (500MHz, DMSO-d 6 )δ9.07(d, J=1.7Hz, 2H), 9.00(d, J=5.1Hz, 2H), 8.57(d, J=16.6Hz, 2H), 8.33(dd, J=5.1, 1.7Hz, 2H), 8.07–7.98(m,4H), 7.97–7.93(m,2H), 7.75–7.66(m,4H), 4.29(s,6H), 1.86(s,12H).
Embodiment 2
[0043] The synthesis of bpySN compound, its structural formula is:
[0044]
[0045] The preparation method of bpySN is as follows:
[0046] A mixture of 2,2'-bipyridyl-4,4'-dicarbaldehyde (0.106g, 0.5mmol) and 3-ethyl-2-methylbenzothiazolium iodide (0.305g, 1mmol) in pyridine / ethanol (16mL; 1:15v / v) was heated to 80°C, reacted in the dark under an argon atmosphere for 19 hours, and then the reaction was lowered to room temperature. The precipitate was filtered and washed with ether, and the obtained crude product was recrystallized from ethanol to obtain orange Yellow crystals, and the obtained crystals were further dried to obtain 0.224 g of orange powder, with a yield of 57%. Characterized by NMR, its molecular formula is C 32 h 28 I 2 N 4 S 2 , 1 H NMR (400MHz, DMSO-d 6 )δ8.99–8.96(m,2H),8.55(d,J=8.1Hz,2H),8.43–8.40(m,4H),8.20(dd,J=5.2,1.7Hz,2H),7.99–7.85 (m, 4H), 5.09 (q, J = 7.1 Hz, 4H), 1.53 (t, J = 7.2 Hz, 6H).
Embodiment 3
[0048] The synthesis of bpyPCN compound, its structural formula is:
[0049]
[0050] The preparation method of bpyPCN is as follows:
[0051] A mixture of 2,2'-bipyridyl-4,4'-dicarbaldehyde (0.106g, 0.5mmol) and 1-ethyl iodide 2,3,3-trimethylbenzindole (0.365g, 1mmol) Heated to 80°C in pyridine / ethanol (16mL; 1:15v / v), reacted in the dark under an argon atmosphere for 19 hours, then cooled the reaction to room temperature, filtered and washed the precipitate with ether, and filtered the obtained crude product through ethanol Red-brown crystals were obtained by recrystallization, and the obtained crystals were further dried to obtain 0.294 g of red-brown powder, with a yield of 65%. Characterized by NMR, its molecular formula is C 46 h 44 I 2 N 4 , 1 H NMR (500MHz, DMSO-d 6 )δ9.11(d, J=1.6Hz, 2H), 9.03(d, J=5.1Hz, 2H), 8.71(d, J=16.5Hz, 2H), 8.51(d, J=8.4Hz, 2H) ,8.42–8.35(m,4H),8.30–8.22(m,4H),8.07(d,J=16.6Hz,2H),7.87(ddd,J=8.3,6.8,1.4Hz,2H),7.80(t , J=7.6Hz, 2H), 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com