Lithium-rich manganese-based cathode material and preparation method thereof, positive electrode piece and lithium ion secondary battery
A positive electrode material, lithium-rich manganese-based technology, applied in the field of lithium-ion secondary batteries, can solve the problem of low charge and discharge efficiency in the first phase, and achieve high energy density, simple process, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
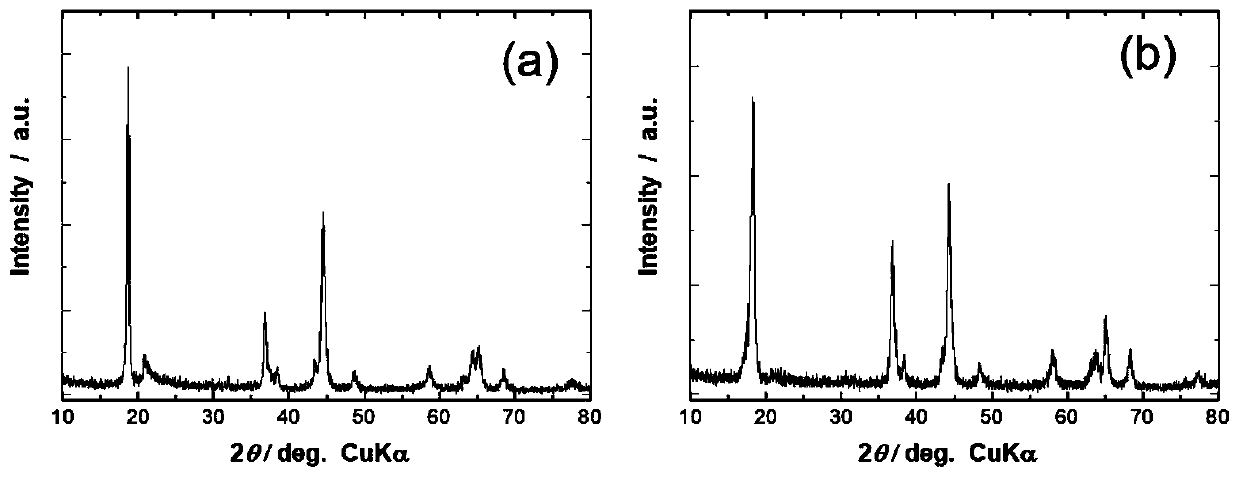
Image
Examples
Embodiment 1
[0092] Synthesis of Sodium-Containing Layered Composite Metal Oxide Precursor Na 0.8 Li 0.12 Ni 0.22 mn 0.66 o 2 .
[0093] The method is, a certain amount of Na 2 CO 3 , Li 2 CO 3 , Mn(CH 3 COO) 2 4H 2 O,Ni(CH 3 COO) 2 4H 2 O, mix the raw materials in a mortar after weighing, then heat to 300°C in a pre-fired furnace to obtain a gel-like mixture, continue to heat at 300°C for 4 hours, cool and pulverize in the grinding and grind. Then the mixture is poured into a ball mill jar, and the fully mixed powder is mixed by ball milling, pressed into a tablet under a pressure of 10-20Mpa, then put into a refractory crucible, and calcined in air at a temperature of 750°C for 12 Hour. After natural cooling, crushing and grinding, the powdery precursor Na 0.8 Li 0.12 Ni 0.22 mn 0.66 o 2 .
[0094] The precursor Na prepared by the above method 0.8 Li 0.12 Ni 0.22 mn 0.66 o 2 in LiNO 3 -LiI medium for ion exchange.
[0095] The specific method is to add the pr...
Embodiment 2
[0108] This example synthesizes the sodium-containing layered composite metal oxide precursor Na 1.0 Li 0.2 Ni 0.2 mn 0.6 o 2 , and perform ion exchange.
[0109] Precursor Na 1.0 Li 0.2 Ni 0.2 mn 0.6 o 2 The synthesis method is basically the same as that described in Example 1. According to Na 1.0 Li 0.2 Ni 0.2 mn 0.6 o 2 composition, adjust Na 2 CO 3 , Li 2 CO 3 , Mn(CH 3 COO) 2 4H 2 O,Ni(CH 3 COO) 2 4H 2 The proportion of raw materials such as O.
[0110] At the same time, the precursor Na prepared by the above method 0.8 Li 0.12 Ni 0.22 mn 0.66 o 2 Prepare lithium-rich manganese-based positive electrode material Li by the ion exchange method described in Example 1 1.2 Ni 0.2 mn 0.6 o 2 .
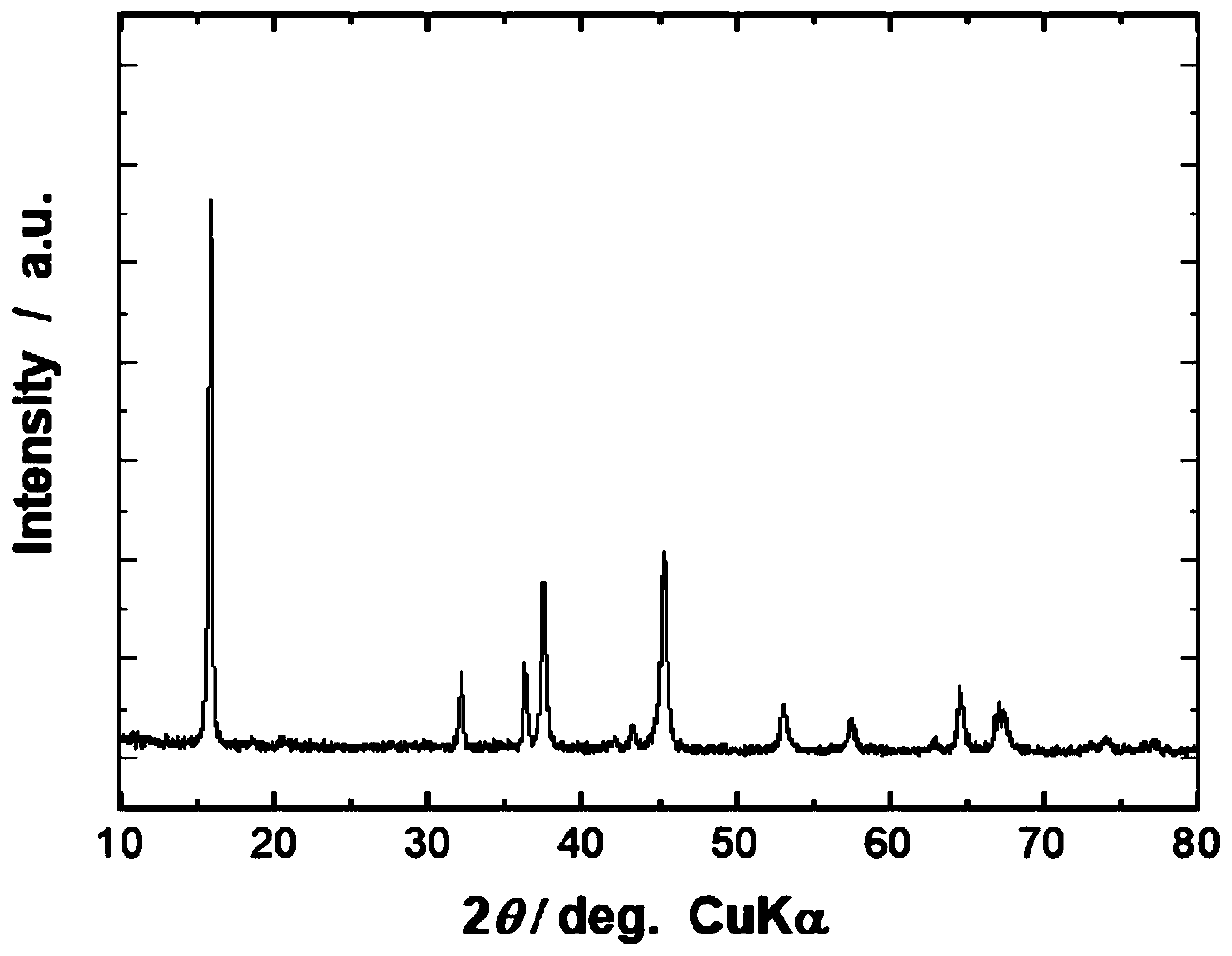
[0111] The precursor Na obtained in this example 1.0 Li 0.2 Ni 0.2 mn 0.6 o 2 The measurement results of the synthetic powder X-ray diffraction measurement show that: the precursor Na 0.8 Li 0.12 Ni 0.22 mn 0.66 o 2 The crystal structure belong...
Embodiment 3
[0114] This example is synthesized with sodium-containing layered composite metal oxide P2 type Na 0.67 Li 0.08 Ni 0.23 mn 0.69 o 2 Precursors for ion exchange.
[0115] Among them, P2 type Na 0.67 Li 0.08 Ni 0.23 mn 0.69 o 2 The synthesis method of the precursor is basically similar to that of the P3 type precursor described in Example 1. Due to the high synthesis temperature of the P2 compound, the sintering conditions were adjusted to sinter at 900 for 24 hours. All the other steps are the same as in Example 1.
[0116] The P2 type Na prepared by the above method 0.67 Li 0.08 Ni 0.23 mn 0.69 o 2 Precursor is prepared cathode material Li by the ion exchange method described in embodiment 1 1.08 Ni 0.23 mn 0.69 O.
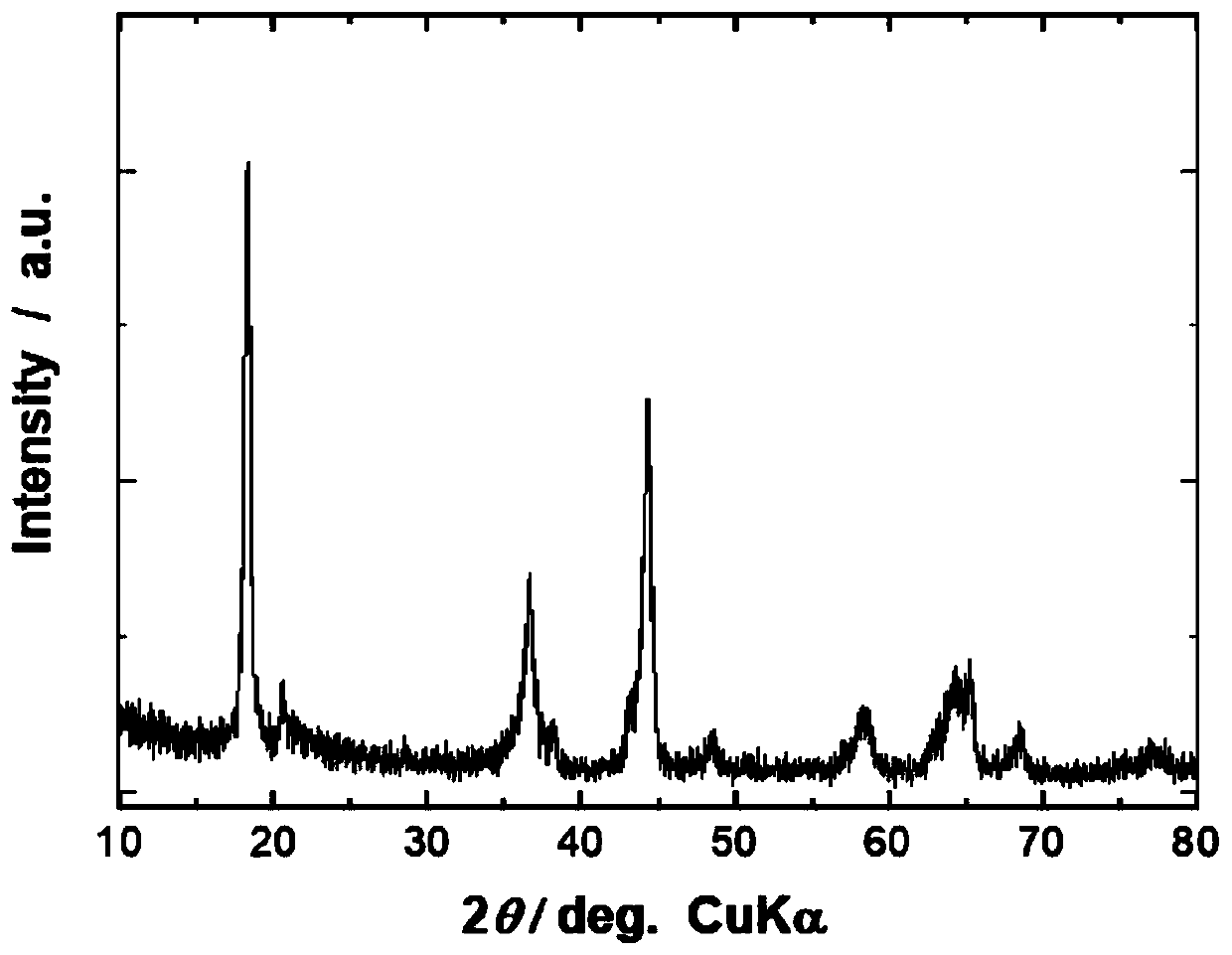
[0117] Na synthesized in this example 0.67 Li 0.08 Ni 0.23 mn 0.69 o 2 The crystal structure of the precursor belongs to the P2 type layered structure. In addition, no other impurities were observed, and it belonged to a single phase.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com