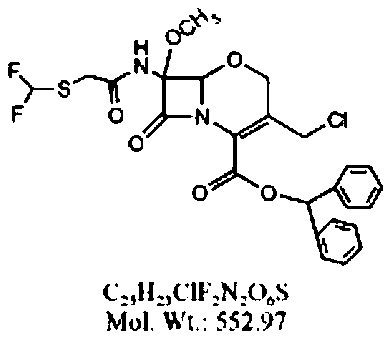
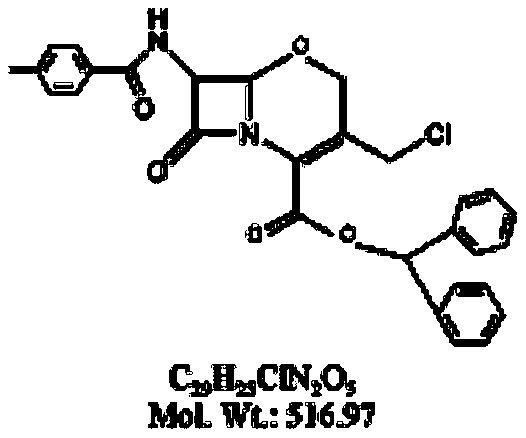
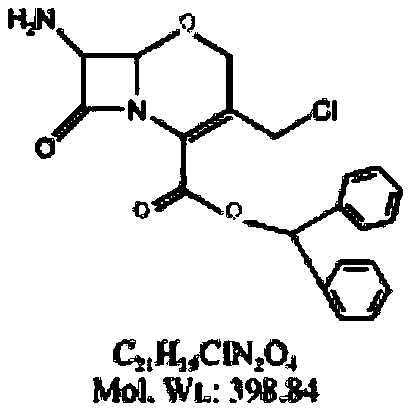
Preparation method of flomoxef parent nucleus
A technology of Oxycephem nucleus and Fluoxefem, which is applied in the field of medicine and chemical industry, can solve the problems of hindering bacterial cell wall biosynthesis and less research, and achieve the effects of low production cost, easy industrialization, and simple synthesis operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A preparation method of fluoxycephalosporin nucleus, comprising the following preparation steps:
[0033] (1) Add oxycephem nuclei and chloroform to the reaction vessel, stir until the oxycephem nuclei are completely dissolved, add organic base and phosphorus pentachloride to the reaction feed liquid, and control the reaction temperature at -10 to 10°C , as the reaction proceeds, naturally heat up to 0-5°C, keep warm, and the reaction time is 1.5-5h;
[0034] (2), after the reaction of step (1) is completed, add methanol to the reaction feed liquid and control the temperature at 0-5°C, stir the reaction feed liquid, and the reaction time is 1.5-5h;
[0035] (3) After the reaction in step (2), add purified water to the reaction feed liquid, stir the reaction feed liquid for 5 to 15 minutes, let it stand for stratification, take the chloroform phase, add sodium sulfate to the chloroform phase to dry , obtain the reaction feed liquid containing intermediate 1 after filtra...
Embodiment
[0046] Example: Add 10.00 g (0.0193 mol) of oxycephem parent nucleus and 50 mL of chloroform into the reaction vessel, stir until the oxycephem parent nucleus is completely dissolved, control the temperature of the reaction feed liquid to -10 ° C, and add Add quinoline 2.74g (0.0213mol), then add phosphorus pentachloride 4.23g (0.0203), as the reaction proceeds, the reaction feed liquid naturally warms up to 0 ~ 5 ℃, heat preservation, the reaction time is 2h, after the reaction, Add 50ml of methanol to the reaction feed liquid, control the reaction temperature at 0-5°C, keep warm, stir for 3 hours, take a sample for detection, after the reaction is complete, add 100 ml of purified water to the reaction feed liquid, stir for 5 minutes, let stand for stratification, and take chloroform Add 1g of sodium sulfate to dry, filter to obtain the reaction feed liquid containing intermediate 1, add tert-butyl hypochlorite 4.20g (0.0387mol) to the reaction feed liquid, then add dropwise t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com