N-doped microporous carbon sphere ORR catalytic material and preparation method and application thereof
A catalytic material and microporous carbon technology, applied in the field of electrocatalysis, can solve problems such as unfavorable large-scale commercial preparation and application, high experimental conditions, and inability to meet requirements, etc., to promote the adsorption of oxygen molecules and further catalytic reactions and equipment requirements. Not high, the effect of improving structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
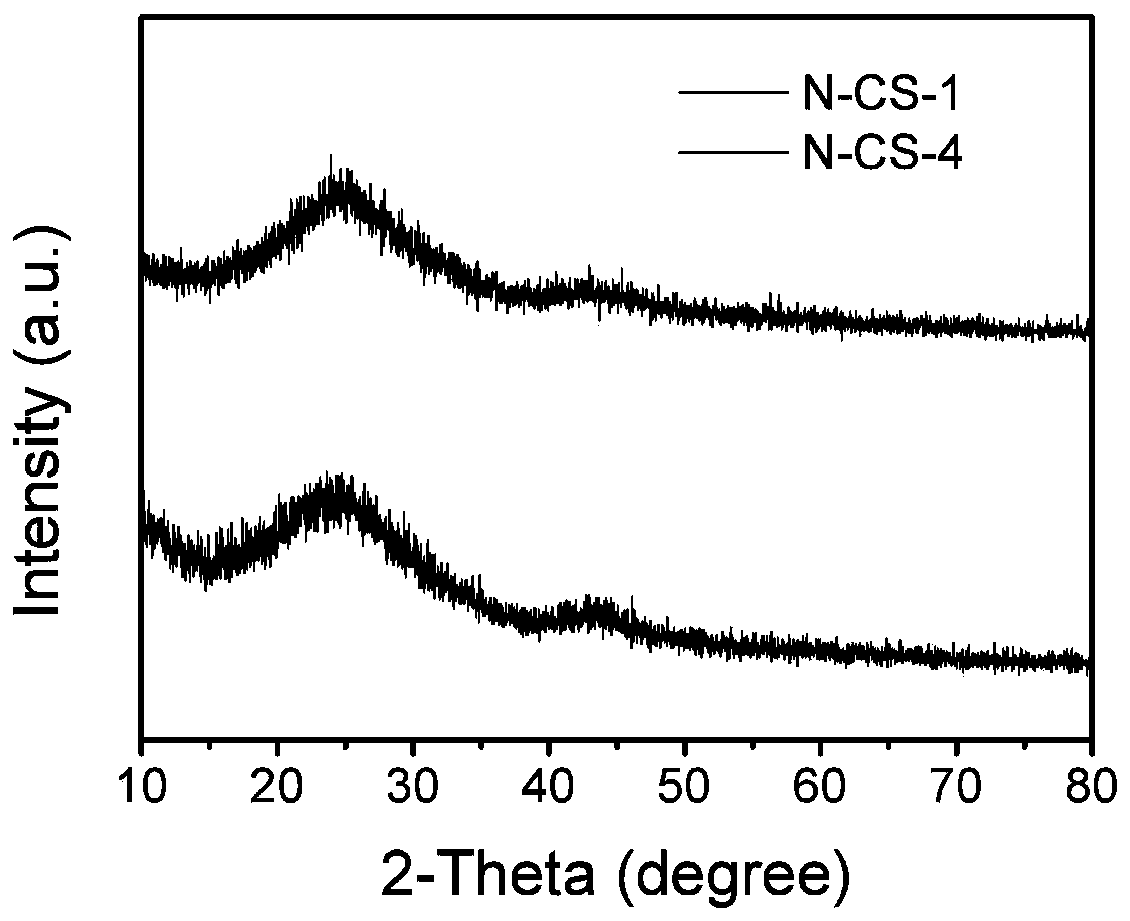
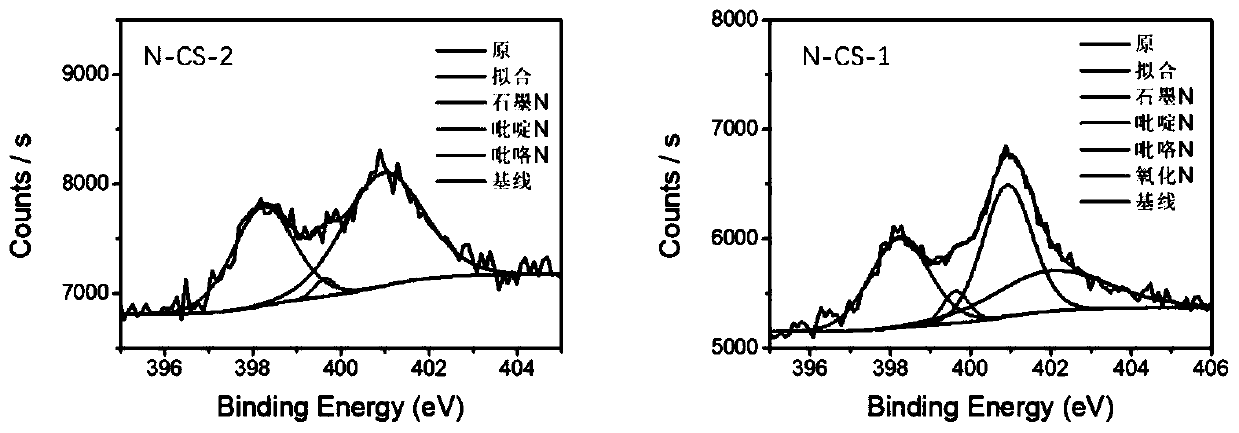
[0052] First, add 1.36g of zinc chloride into 5mL of formaldehyde (0.03mol) solution, and stir for 0.5h under ice-bath conditions to completely dissolve the zinc salt to form a clear high-salt solution. 1 mL of pyrrole monomer (0.015 mol) was added to the above solution, and after stirring for 0.5 h at 0-5° C., a uniform yellow oily liquid was formed. Transferred to the environment of 30° C. and allowed to stand for reaction for 12 hours, forming a black pyrrole zinc gel solid. Then dry in an oven at 105 °C for 24 h to evaporate water, crush the precursor into powder, place it in a porcelain boat, and then carry out high-temperature pyrolysis in a tube furnace filled with argon atmosphere, with a heating rate of 3 °C / min, controlled The temperature was kept at 900°C for 2h. The sample obtained by the above steps was named N-CS-1. The N doping content is 2.94%; the relative content of pyridine nitrogen is 36.8%
[0053] The micropore porosity is 87.7%, and the specific surfa...
Embodiment 2
[0056] First add 3.4g of zinc chloride into 5mL of formaldehyde (0.03mol) solution, and stir for 0.5h under ice bath conditions to completely dissolve the zinc salt to form a clear high-salt solution. 1 mL of pyrrole monomer (0.015 mol) was added to the above solution, and after stirring for 0.5 h at 0-5° C., a uniform yellow oily liquid was formed. Transferred to the environment of 30° C. and allowed to stand for 6 hours to react, forming a black pyrrole zinc gel solid. Then dry in an oven at 105 °C for 24 h to evaporate water, crush the precursor into powder, place it in a porcelain boat, and then carry out high-temperature pyrolysis in a tube furnace filled with argon atmosphere, with a heating rate of 3 °C / min, controlled The temperature was kept at 900°C for 2h. The sample obtained by the above steps was named N-CS-2. N doping content is 2.19%; relative pyridine nitrogen content is 51.5%
[0057] The micropore porosity is 89.7%, and the specific surface area is 1689m ...
Embodiment 3
[0060] First add 2g of zinc chloride to 10mL of formaldehyde (0.08mol) solution, and stir for 0.5h under ice bath conditions to completely dissolve the zinc salt to form a clear high-salt solution. 1.4 mL of pyrrole monomer (0.02 mol) was added to the above solution, and after stirring for 0.5 h at 0-5° C., a uniform yellow oily liquid was formed. Transferred to the environment of 30° C. and allowed to stand for reaction for 12 hours, forming a black pyrrole zinc gel solid. Then dry in an oven at 105 °C for 24 h to evaporate water, crush the precursor into powder, place it in a porcelain boat, and then perform high-temperature pyrolysis in a tube furnace filled with argon atmosphere, with a heating rate of 2 °C / min, controlled The temperature is kept at 1100°C for 2h. The sample obtained by the above steps is named N-CS-3
[0061] The obtained sample was used as the cathode material of an aluminum-air battery, and the electrochemical performance test was the same as in Examp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com