Lepidolite ore and sodium sulfate roasting and leaching method
A technology of lepidolite and sodium sulfate, which is applied in the field of lithium extraction from ores, can solve the problems of low leaching rate of rubidium and cesium, high reagent cost, melting, etc., and achieve the effects of reducing lithium concentration energy consumption, increasing calcination conversion rate, and improving sintering performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
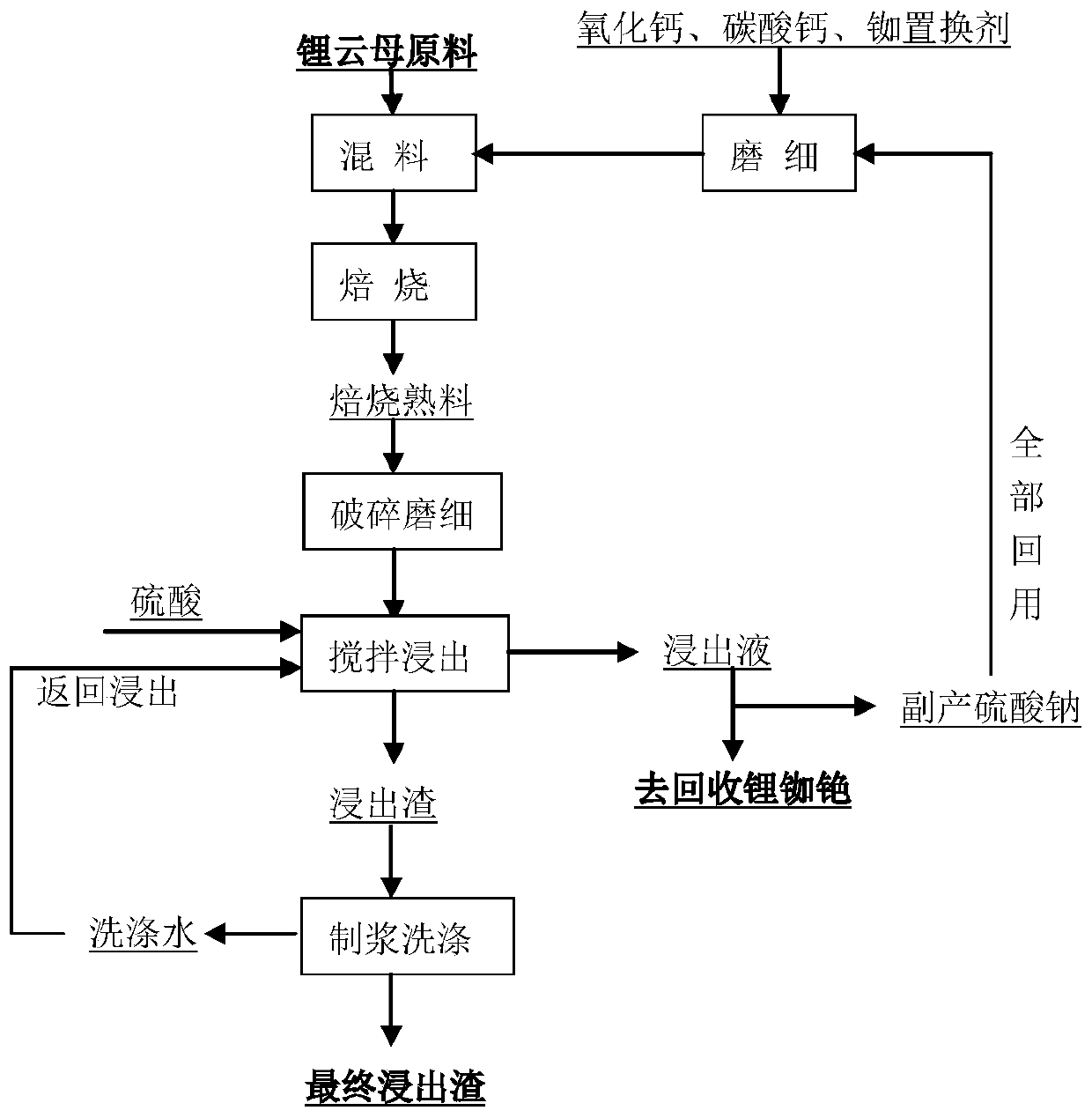
[0078] Proceed as follows:
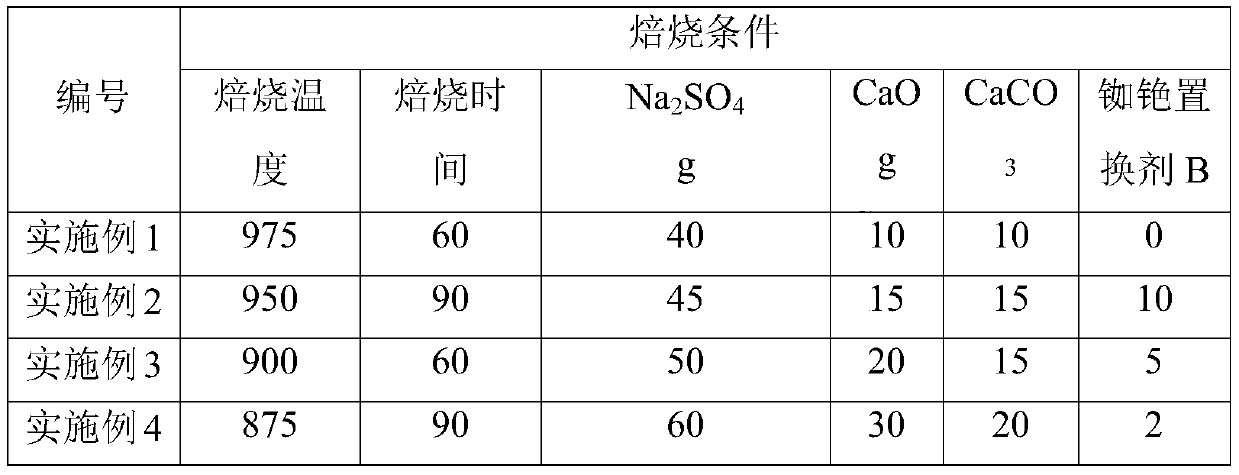
[0079] ① Take 100g lepidolite concentrate and weigh the following reagents: 10g K 2 SO 4 , 30g Na 2 SO 4 , 5g CaO, 5gCaCO 3 , 3g of rubidium and cesium displacer, the additives are mixed and ground to more than 50% and less than 200 mesh;
[0080] ②Mix the lepidolite concentrate and the finely ground reagent evenly;
[0081] ③Put the mixed material in a crucible and bake at 975°C for 60 minutes;
[0082] ④ Grind the calcined sand until 90% of it is less than -100 mesh;
[0083] ⑤Transfer the finely ground calcine to the leaching tank for leaching, the leaching agent is 20g / L sulfuric acid solution
[0084] 100ml, temperature 60°C, stir for 90min, filter;
[0085] ⑥ Pulping and washing, adding 150ml of water to the leaching residue to make pulp, stirring and washing, temperature 50°C, when stirring and washing
[0086] Wait for 30 minutes, filter, and return the obtained washing water to add acid, then stir and leach.
[0087] The lithium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com