A kind of jatrorrhizine platinum (ii) complex targeting human bladder cancer and its synthesis method and application
A bladder cancer, jatrorrhizine technology, applied in platinum-based organic compounds, platinum-based organic compounds, drug combinations, etc., can solve problems such as no related reports, achieve high yield, superior in vitro and in vivo antitumor activity and target The effect of tropism, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
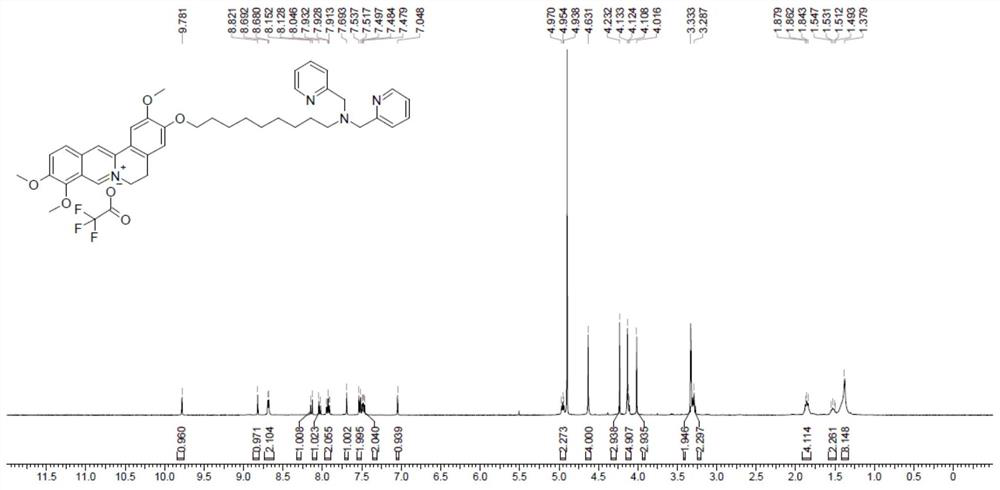
[0036] Weigh 1.0 mol of derivative 1, 0.8 mol of potassium iodide and 1.50 mol of lutidine amine in a round-bottomed flask, add 5.0 ml of acetonitrile, the added solvent is just enough to dissolve the solid, and make the reaction more fully. After reacting for 4 hours, the J-TFA ligand was obtained as a yellow solid powder with a yield of 88.9%.
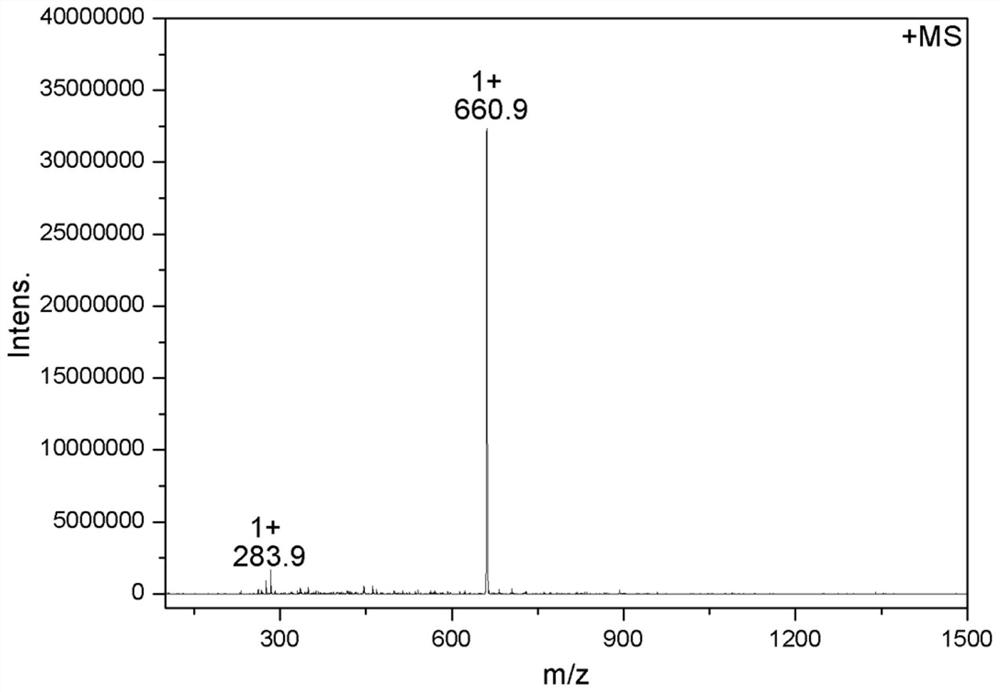
[0037] Add 1.0 mol ligand J-TFA and 1.0 mol dichlorobis(dimethylsulfoxide) platinum(II) into a 100.0 mL round bottom flask, dissolve in 25.0 mL acetonitrile solution, and carry out at 60°C After the coordination reaction for 4.0 h, the product was washed with acetonitrile and dried under vacuum at 45° C. to obtain the yellow target product Pt1. The yield was 93.0%.
[0038] The synthetic route of the jatrorrhizine platinum (II) complex targeting human bladder cancer of the present invention is as follows:
[0039]
Embodiment 2
[0041] The difference from Example 1 is that 1.0mol of ligand J-TFA and 1.0mol of dichlorobis(dimethylsulfoxide)platinum(II) were added to a 100.0mL round-bottomed flask, dissolved in 50.0 In mL acetonitrile solution, the coordination reaction was carried out at 60°C for 4.0h, washed with acetonitrile and then vacuum-dried at 45°C to obtain the yellow target product Pt1. The yield was 85.2%.
Embodiment 3
[0043] The difference from Example 1 is that 1.0mol of ligand J-TFA and 1.0mol of dichlorobis(dimethylsulfoxide)platinum(II) were added to a 100.0mL round bottom flask, dissolved in 5.0 In mL acetonitrile solution, the coordination reaction was carried out at 60°C for 4.0h, washed with acetonitrile and then vacuum-dried at 45°C to obtain the yellow target product Pt1. The yield was 90.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com