A kind of preparation method and application of amino acid stannous complex
An amino acid stannous and complex technology, which is applied in the preparation of organic compounds, the preparation of cyanide reactions, organic compounds/hydrides/coordination complex catalysts, etc. Industrial production and other problems, to achieve the effect of high yield, high molecular weight of the product, and high syndiotactic selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
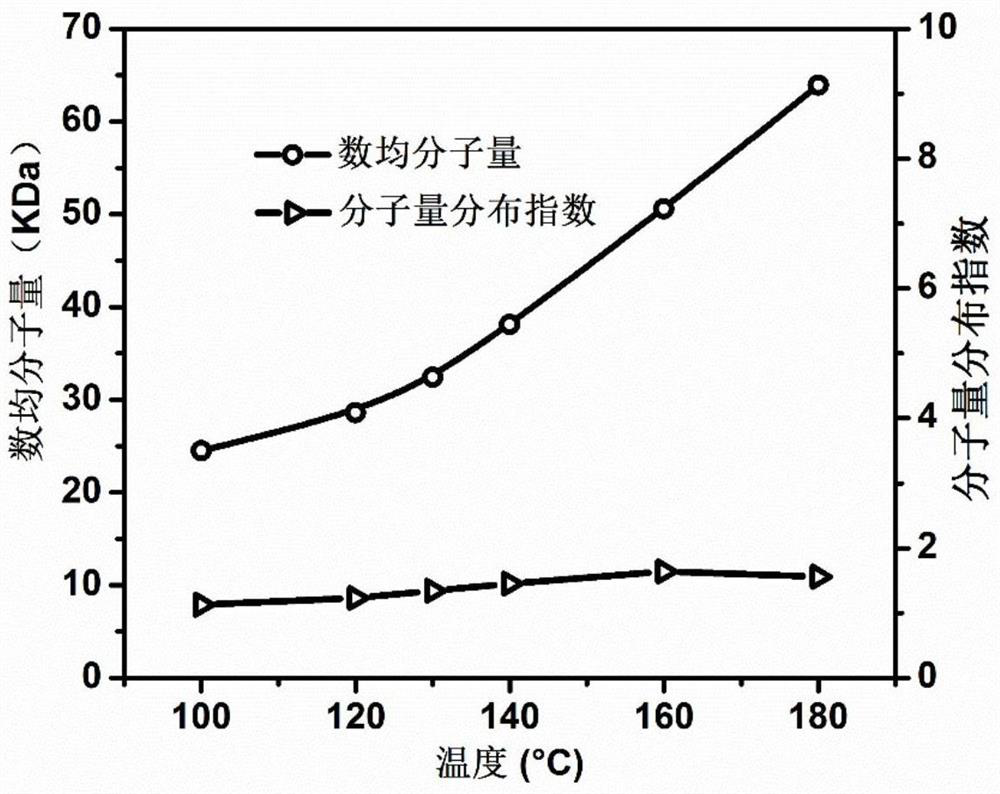
Examples
Embodiment 1
[0033] The synthesis of embodiment 1 catalyst L-tin protophenylalanate
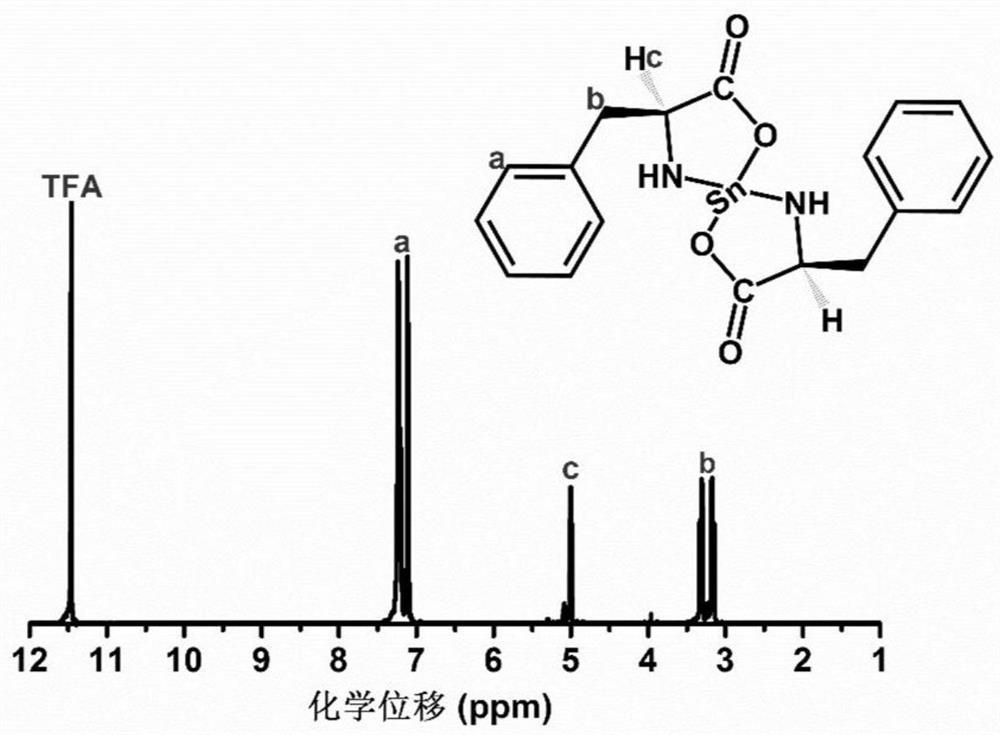
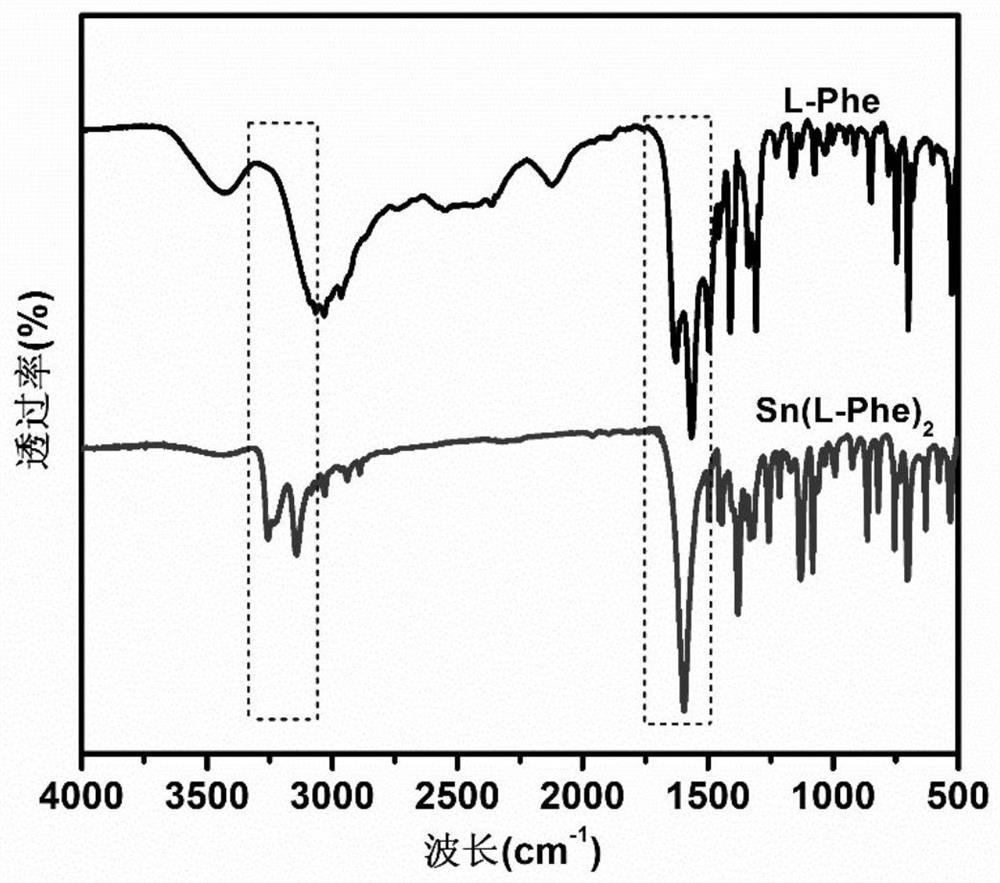
[0034] Add L-phenylalanine (9.9g, 0.06mol) and methanol (40ml) into a 100ml three-neck flask, stir and react in a constant temperature water bath at 60°C for 20min, then add triethylamine solution (6.07g, 0.06mol) dropwise, Add dropwise in 30 minutes, L-phenylalanine is all dissolved, then feed nitrogen into the solution for 10min, remove the oxygen in the solution and the reaction flask, and add stannous chloride (SnCl 2 2H 2 (2, 6.78g, 0.03mol) 10ml of methanol solution, after 30 minutes of dropwise addition, react at 60°C for 5 hours, the L-stannous phenylalanine is precipitated in granular form, filtered, and the product is dried in a vacuum oven for 24 hours , to obtain 12.4g, yield 12.4 / 13.38×100%=92.68%. Elemental analysis value (composition C 18 h 20 N 2 o 4 Sn) (%) C: 48.43 (47.23), H: 4.48 (4.42), Sn: 26.46 (26.53), (measured in air).
Embodiment 2
[0035] The synthesis of embodiment 2 catalyst L-stannous leucine
[0036] The experimental procedure was the same as in Example 1, except that L-phenylalanine was replaced with 0.06 mol L-leucine to obtain 10.82 g of stannous L-leucine with a yield of 95.33%. Elemental analysis value (composition C 12 h 24 N 2 o 4 Sn) (%) C: 38.05 (37.93), H: 6.34 (6.21), Sn: 31.22 (30.83), (measured in air).
Embodiment 3
[0037] The synthesis of embodiment 3 catalyst L-stannous isoleucine
[0038] The experimental procedure was the same as that in Example 1, except that L-phenylalanine was replaced with 0.06 mol L-isoleucine to obtain 9.93 g stannous L-isoleucine with a yield of 87.49%. Elemental analysis value (composition C 12 h 24 N 2 o 4 Sn) (%) C: 38.05 (37.81), H: 6.34 (6.26), Sn: 31.22 (29.87), (measured in air).
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com