Labdane diterpene derivatives as well as pharmaceutical composition and application thereof
The technology of a derivative and a composition is applied in the field of helichanoid diterpene derivatives and pharmaceutical compositions thereof, which can solve the problems of high toxicity and poor activity, and achieve the effect of eliminating interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the extraction method of helianthane type diterpene derivatives, the specific steps are as follows:
[0048] (1) Dry and pulverize the Leonurus branch;
[0049] (2) At room temperature, the motherwort sticks pulverized in step (1) were extracted 3 times with an aqueous ethanol solution with a volume fraction of 85%, each extraction was 24 hours, the ethanol extract was combined, filtered, and the filtrate was concentrated under reduced pressure get the extract;
[0050] (3) suspending the medicinal extract of step (2) in water, and extracting with sherwood oil, ethyl acetate and n-butanol successively to obtain sherwood oil extract, ethyl acetate extract and n-butanol extract;
[0051] (4) The ethyl acetate extract of step (3) is concentrated under reduced pressure to reclaim the solvent to obtain the ethyl acetate extract, then the ethyl acetate extract is dissolved in chloroform and adsorbed on silica gel, and the solvent chloroform is evaporated and gr...
Embodiment 2
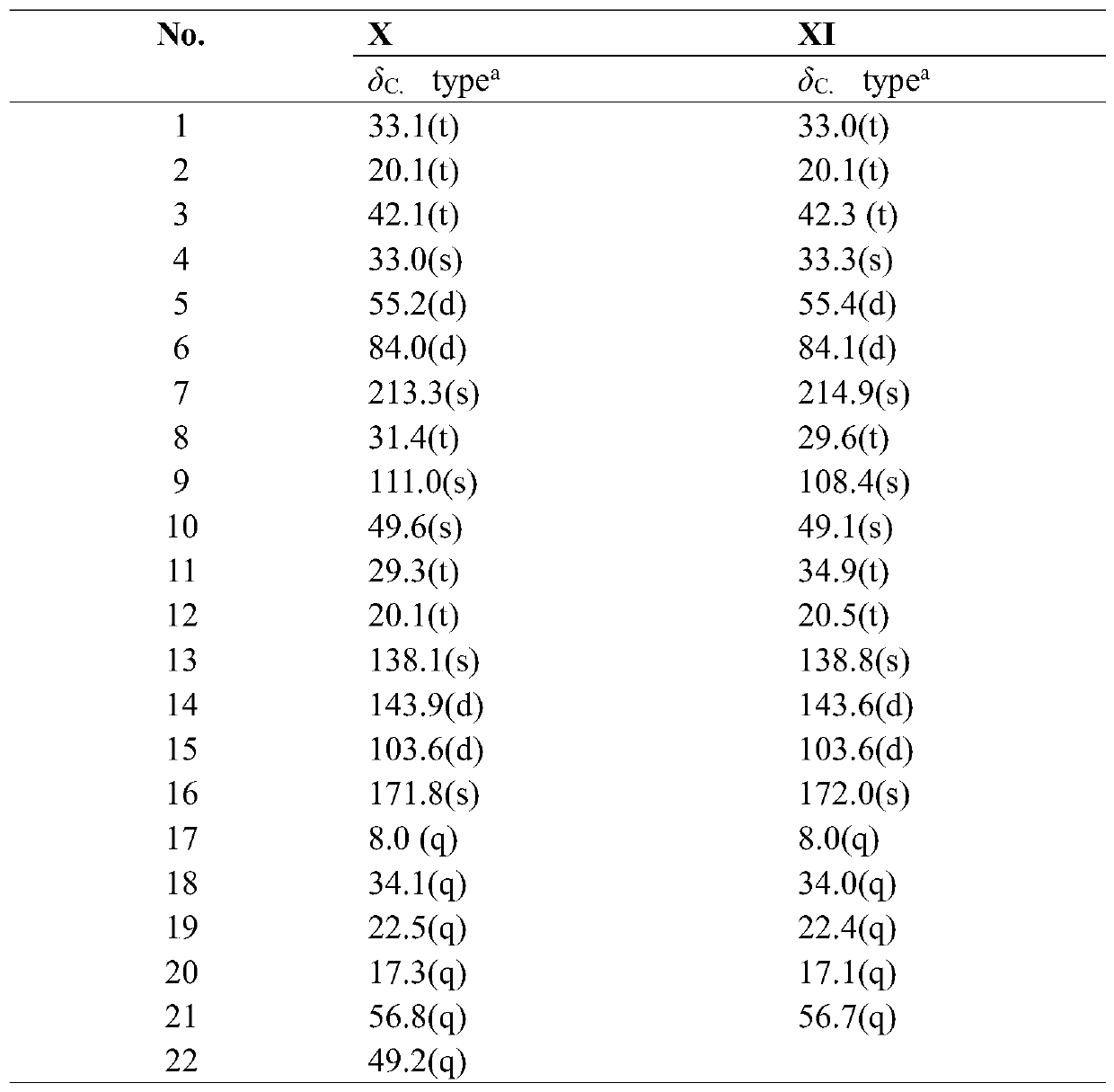
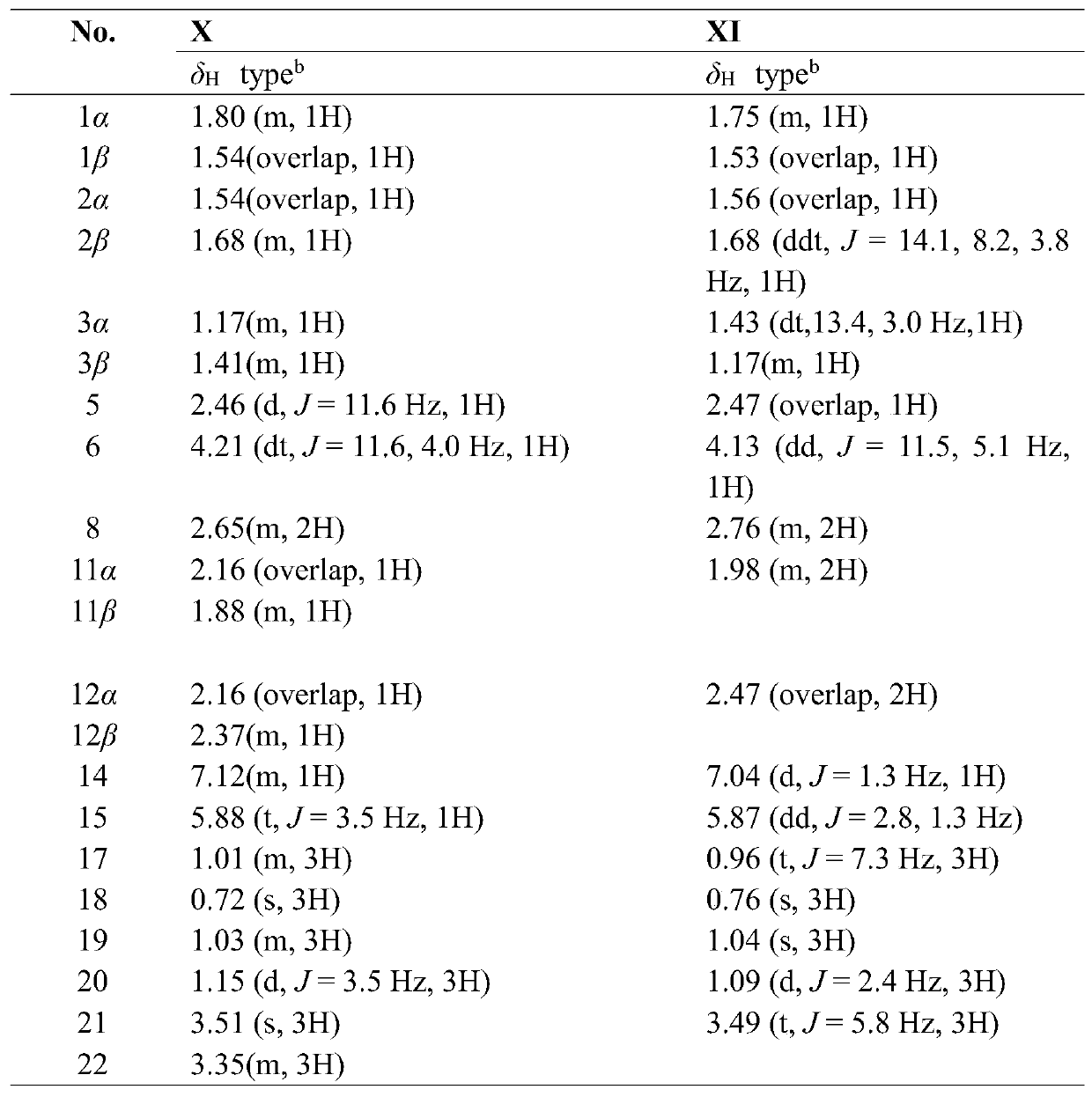
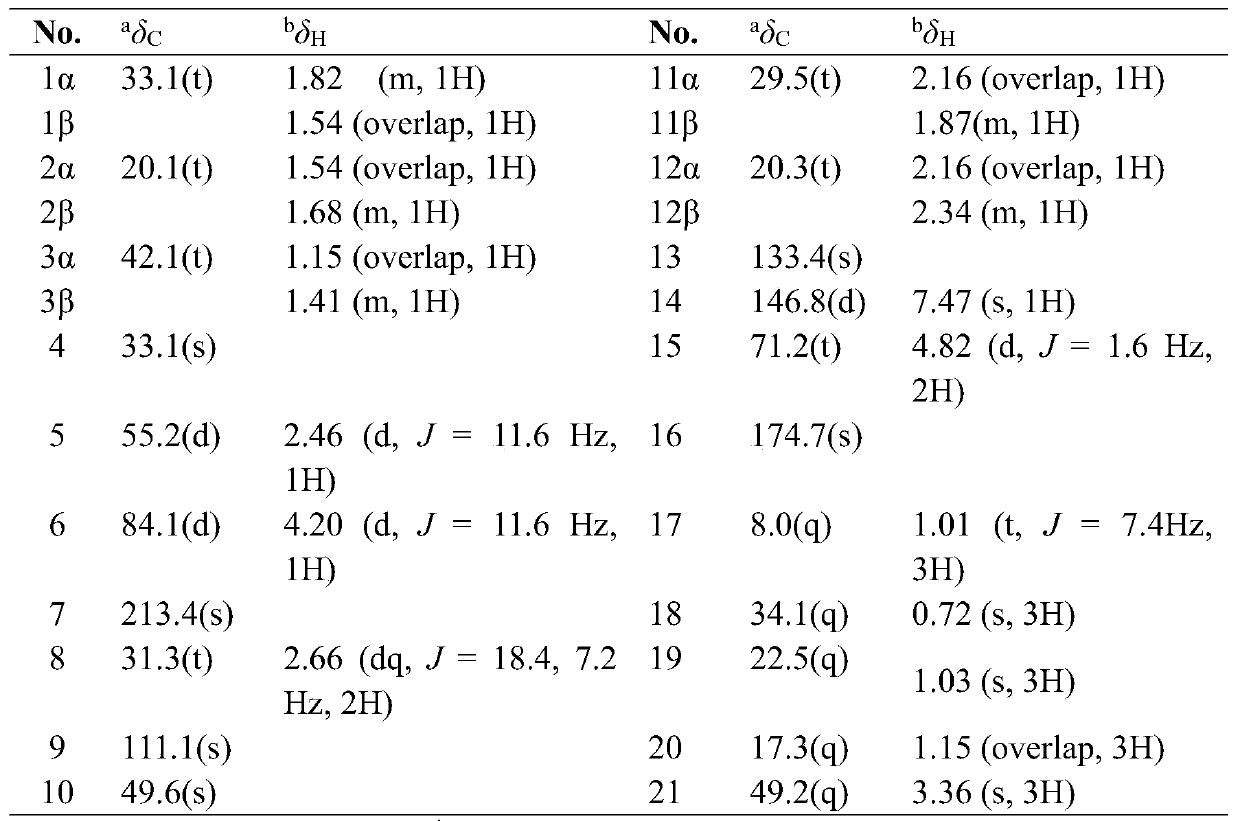
[0125] Embodiment 2: The structural formula of embodiment 1 is the pharmacological experiment of I~XII helianthin type diterpene derivatives;
[0126] Cell culture: RAW264.7 cells (ATCC) gradually converge after growing for a period of time and cover the bottom of the bottle to form a monolayer cell membrane. When the coverage of adherent cells reaches 70-80% (about 1-2 days); Wash the cells once with PBS solution (Gibco fetal bovine serum), add 1 mL of trypsin (Gibco), digest at room temperature for 5 min, add 10 mL of DMEM complete medium containing 10% fetal bovine serum, blow off the cells, and culture in a cell incubator;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com