Application of pyrazolidinyl compounds in preparation of preparations used for treating dermatitis
A technology of pyrazolidine and compounds, applied in the application field of pyrazolidine compounds in the preparation of preparations for treating dermatitis, which can solve problems such as drug resistance, recurrence of dermatitis symptoms, and reddened skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of above-mentioned emulsifiable paste is as follows:
[0039] A (oil phase): Weigh or measure white vaseline, glyceryl monostearate, stearic acid, ethylparaben, 2,6 tert-butyl p-phenol and liquid paraffin into a No. 1 beaker, and put 1 The beaker was heated to 80°C and stirred to completely dissolve the substances.
[0040] B (water phase): Weigh or measure distilled water, glycerin, triethanolamine, and sodium 12 alkylsulfonate respectively into the No. 2 beaker, heat the No. 2 beaker to 75° C. and stir to completely dissolve each substance.
[0041] C: Dissolve PY-41 in an appropriate amount of ethanol, slowly add A (oil phase) into B (water phase), stir in the same direction while adding, wait until the temperature drops to 60°C, add C, and stir evenly to room temperature to obtain the PY-41 drug cream.
Embodiment 1
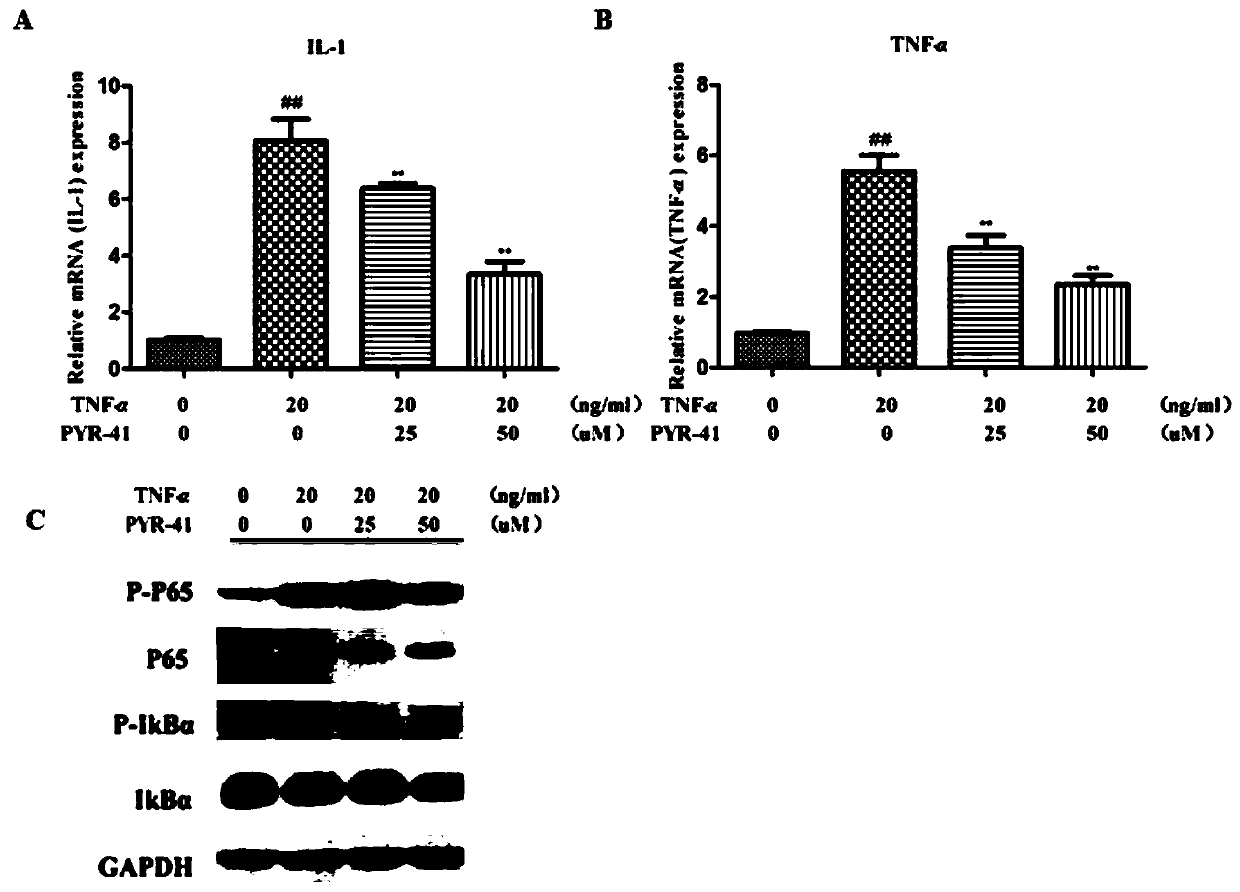
[0044]The HaCaT cells cultured in vitro were stimulated with TNF-α for 1 hour, and then treated with PYR-41 for 6 hours, the cells were collected, total RNA was extracted, and the levels of pro-inflammatory cytokines TNF-α and IL-1 were detected by Real-time PCR , and found that: PYR-41 can dose-dependently reduce the expression of TNF-α and IL-1 within the dose range of 0-50uM.
[0045] HaCaT cells cultured in vitro were stimulated with TNF-α for 30 min, and then HaCat cells were treated with PYR-41 (0 μM, 25 μM, 50 μM) in a concentration gradient for 30 min, and Western blot was used to detect the expression of different components in the NF-κB signaling pathway in the cells Condition. Such as figure 1 As shown in C, the results show that PYR-41 can inhibit the phosphorylation of p65 in a concentration gradient and reduce the activation level of IKBA. As the concentration increases, the effect of PYR-41 on reducing the activation level of IKBA is more significant ( figure ...
Embodiment 2
[0047] Induction of dermatitis and eczema: use a hair clipper on the back of the mouse no less than 2×2cm 2 Area shaving. The sensitization was performed one or two days after the skin preparation to reduce the stimulation of the skin preparation on the back of the mice.
[0048] Because MFG (compound dexamethasone acetate cream), hereinafter referred to as MFG, has anti-inflammatory and anti-allergic effects, it is therefore used as the positive drug group in the experiment. On the first day of the experiment (Day 1), the mice were randomly divided into four groups, 5 in each group, namely the normal group (Normal), the model control group (Model), the administration group (PY-41) and the positive drug control group (MGF). The backs of the mice in the model control group, drug treatment group and positive control group were shaved and daubed with 7% DNCB solution 100 μL / mouse for sensitization (50 μL / time), and the normal control group was daubed with the same amount of sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com