Long chain amine-substituted dimethylanilinium compounds, and preparation method, self-assembled structure and use thereof
A technology of dimethylanilinium and compounds, applied in the field of long-chain amine-substituted dimethylanilinium compounds, can solve the problems of improving the duration of local anesthesia, unable to increase the volume of local injection, and unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
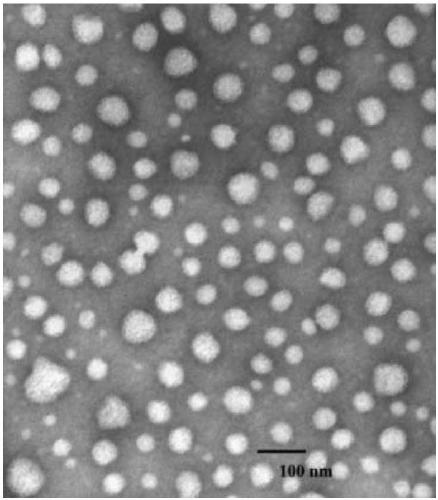
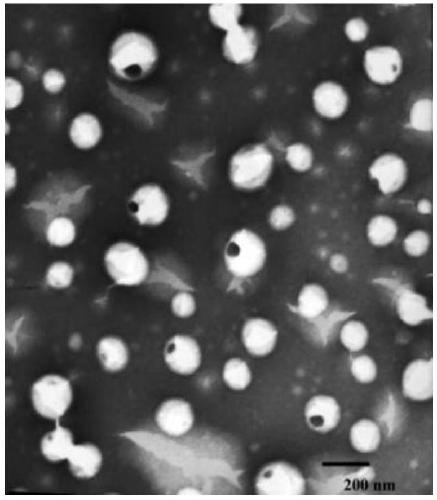
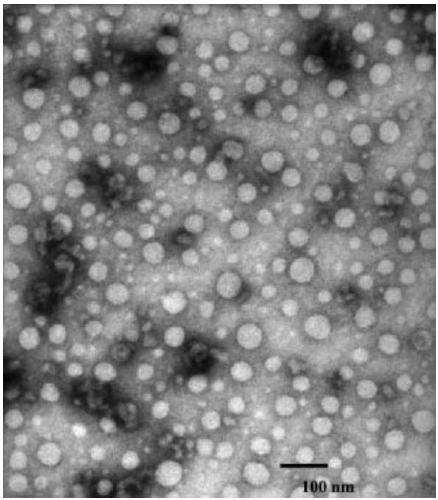
Image
Examples
Embodiment 1
[0051]
[0052] Add 6.0 g of compound (Ⅴ), 1.2 g of pyridine, and 100 mL of dichloromethane into a 200 mL round bottom bottle, and stir well at room temperature. At room temperature, 20 mL of dichloromethane dissolved with 1.1 g of n-butylamine was added dropwise for 15 minutes. Stir at reflux at 40°C for 16h.
[0053] Cool to room temperature, wash with 20 mL x 2 of saturated aqueous sodium bromide, separate the organic phase, and concentrate to dryness. Silica gel column chromatography using dichloromethane:methanol=20:1 as the eluent gave a light yellow colloidal solid with a yield of 32%. 1 H NMR (300MHz, CDCl 3 )δ: 7.02~7.08(m, 3H), 4.80(br, 2H), 4.54(t, J=4.8Hz, 2H), 4.07(t, J=4.8Hz, 2H), 3.70~3.79(m, 4H ), 2.20(s, 6H), 1.33~1.40(m, 4H), 1.30(t, J=4.8Hz, 6H), 0.90(t, J=7.2Hz, 3H). HRMS:[C 20 h 36 N 3 O] +, 334.2853, found 334.2858. As detected by ion chromatography, the bromide ion content is 99.8%.
Embodiment 2
[0055]
[0056] Dissolve 2.0 g of the product of Example 1 in 100 mL of dichloromethane, wash with 20 mL of saturated aqueous sodium chloride solution x 7, separate the organic phase, and concentrate to dryness. Silica gel column chromatography using dichloromethane:methanol=5:1 as the eluent gave a light yellow colloidal solid with a yield of 45%. As detected by ion chromatography, the chloride ion content is 99.6%.
Embodiment 3
[0058]
[0059] Add 3.0 g of the product of Example 1, 0.6 g of pyridine, and 100 mL of dichloromethane into a 200 mL round bottom bottle, and stir evenly at room temperature. At room temperature, 20 mL of a dichloromethane solution in which the amount of iodomethane dissolved in the amount of the example product and the like was added dropwise, and the drop was completed in 15 minutes. Stir at reflux at 40°C for 16h.
[0060] Cool to room temperature, wash with 10 mL x 5 saturated sodium bromide aqueous solution, separate the organic phase, and concentrate to dryness. Silica gel column chromatography with dichloromethane:methanol=20:1 as the eluent gave a light yellow colloidal solid with a yield of 27%. 1 H NMR (300MHz, CDCl 3 )δ: 7.02~7.08(m, 3H), 4.80(br, 2H), 4.54(t, J=4.8Hz, 2H), 4.07(t, J=4.8Hz, 2H), 3.70~3.79(m, 4H ), 2.28(s, 3H), 2.18(s, 6H), 1.33~1.40(m, 4H), 1.30(t, J=4.8Hz, 6H), 0.90(t, J=7.2Hz, 3H). HRMS:[C 21 h 38 N 3 O] + , 348.3009, found 348.3012. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com