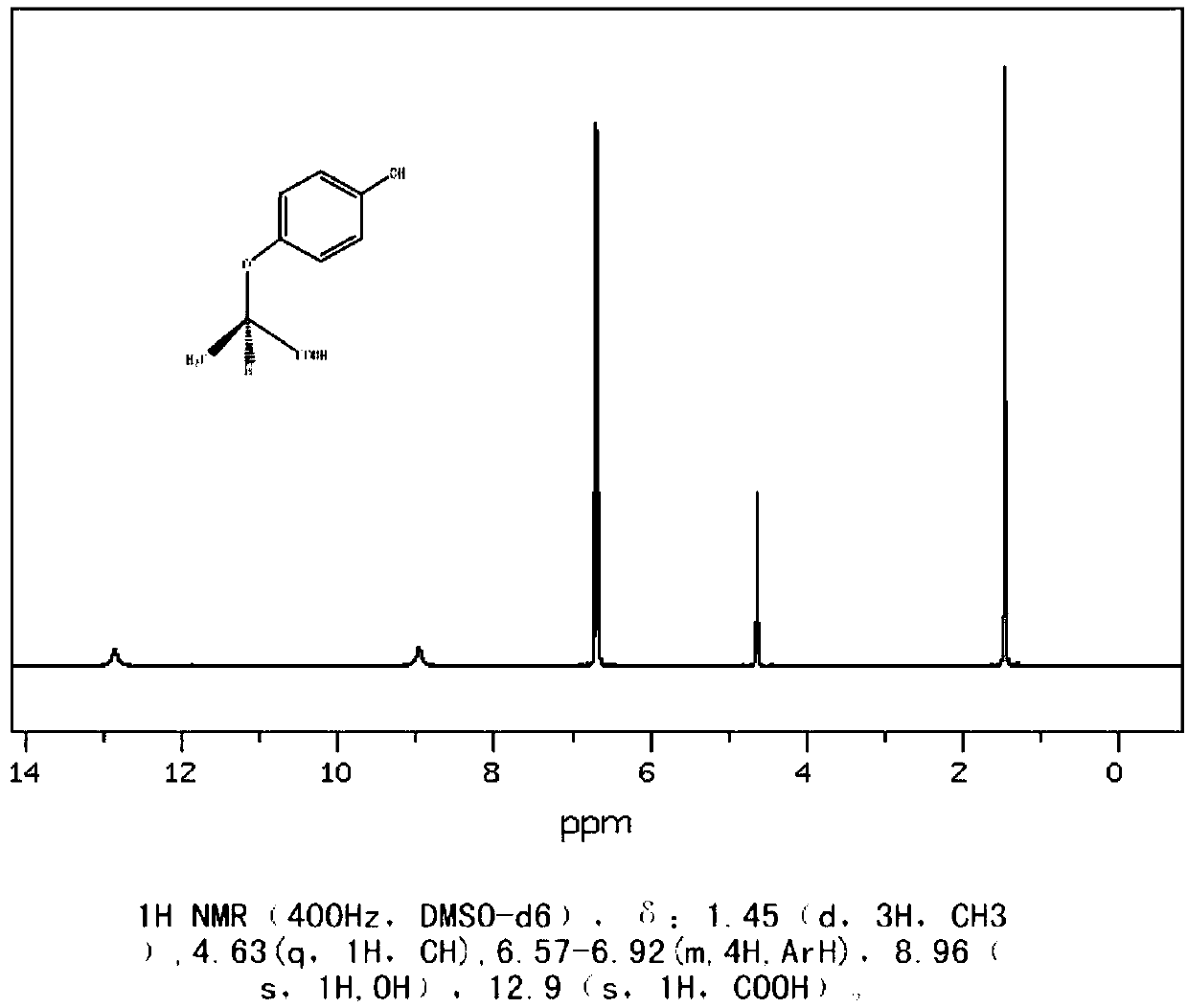
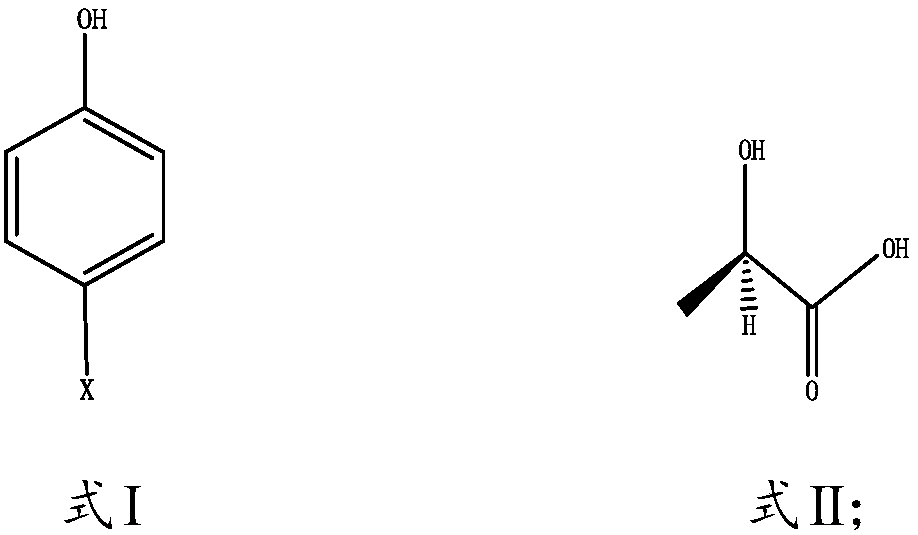R-(+)-2-(4-hydroxyphenoxy)propionic acid preparation method
A technology of hydroxyphenoxy and propionic acid, applied in the field of preparation of R--2-propionic acid, can solve the problems of low production efficiency, long reaction time, difficulty in yeast culture, etc., so as to reduce production costs and avoid polyphenols. Excessive alkylation of impurities, beneficial effect on environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention relates to a kind of preparation method of R-(+)-2-(4-hydroxyphenoxy)propionic acid, comprising the following steps:
[0031] 1) Mix the compound shown in Formula I and Formula II and a solvent in an inert gas environment, and heat for an alkylation reaction; wherein X is F, Cl, Br or I;
[0032]
[0033] 2) After the alkylation and alkylation reaction is completed, add an alkaline solution in a closed environment to adjust the pH to ≥ 8, and separate layers after stirring;
[0034] 3) Separate the lower layer product, add concentrated hydrochloric acid to adjust the pH<1, cool down and crystallize, and recrystallize to obtain R-(+)-2-(4-hydroxyphenoxy)propionic acid;
[0035] 4) Recycling the solvent for reuse.
[0036] According to the present invention, the alkylation reaction is specifically: carrying out the compound represented by formula I, aniline and its derivatives, and the compound represented by formula II according to an equivalent...
Embodiment 1
[0053] R-(+)-2-(4-hydroxyphenoxy)propionic acid is prepared in the presence of aniline and the aniline is recovered.
[0054] step 1
[0055] In 1000mL reaction flask, add p-chlorophenol (64.3g, 0.5mol, 1.0eq), aniline (372.4g, 4.0mol, 8.0eq) and S-2-hydroxypropionic acid (45.04g, 0.5mol, 1.0eq), Nitrogen was replaced three times, the temperature was raised to 50-70° C., and the reaction was kept for 4-6 hours. The conversion rate of S-2-hydroxypropionic acid monitored by HPLC was greater than 99.5%, and the reaction was completed. Cool down, add 30% liquid caustic soda, adjust pH ≥ 8, stir for 30 minutes to separate layers, separate the water layer and adjust pH < 1 with 31% hydrochloric acid, cool to below 10°C, crystallize and filter to obtain R-(+)-2- (4-hydroxyphenoxy)propionic acid crude product 94.5g (undried). The crude product was recrystallized with 94.5 g of water, filtered and dried to obtain 89.1 g of R-(+)-2-(4-hydroxyphenoxy)propionic acid, with a yield of 97....
Embodiment 2
[0059] R-(+)-2-(4-hydroxyphenoxy)propionic acid was prepared using recovered aniline.
[0060] step 1
[0061] Add p-chlorophenol (64.3g, 0.5mol, 1.0eq) to a 1000mL reaction flask, recover aniline (372.4g, 4.0mol, 8.0eq) and S-2-hydroxypropionic acid (45.04g, 0.5mol, 1.0eq) , replaced with nitrogen three times, heated up to 50-70° C., kept for 4-6 hours, and the conversion rate of S-2-hydroxypropionic acid monitored by HPLC was greater than 99.5%, and the reaction was completed. Cool down, add 30% liquid caustic soda, adjust pH ≥ 8, stir for 30 minutes to separate layers, separate the water layer and adjust pH < 1 with 31% hydrochloric acid, cool to below 10°C, crystallize and filter to obtain R-(+)-2- (4-hydroxyphenoxy) propionic acid crude product 93.7g (undried). The crude product was recrystallized with 93.7 g of water, filtered and dried to obtain 88.2 g of R-(+)-2-(4-hydroxyphenoxy)propionic acid, with a yield of 96.8% and a purity of 99.7%.
[0062] step 2
[0063] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com