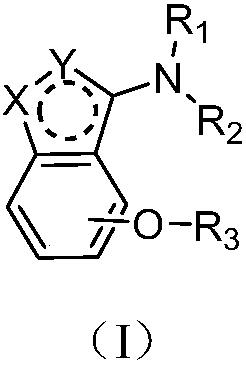
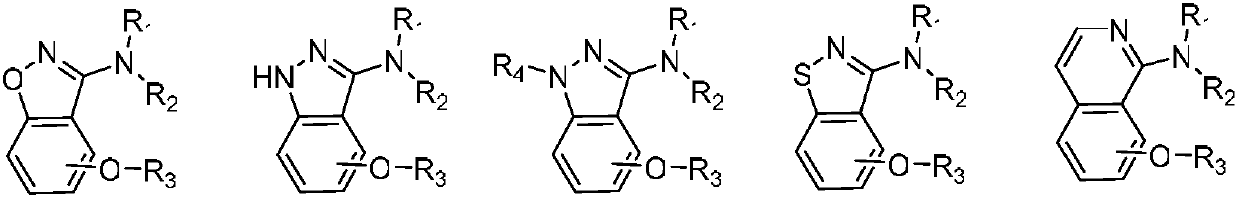
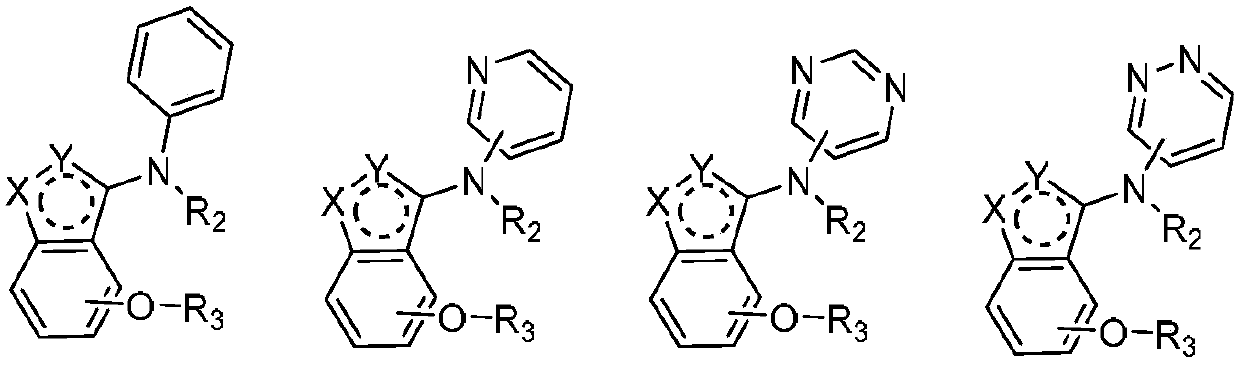
Alkoxy benzo five-membered (six-membered) heterocyclic amine compound and medicinal application thereof
一种烷氧基苯、杂环胺的技术,应用在烷氧基苯并五元(六元)杂环胺类化合物及其药物用途领域,能够解决活性有待进一步提高、抑制作用弱、潜在毒性风险大氰基基团等问题,达到良好潜力和应用前景、抑制活性显著、理化性质理想的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of 4-(2-ethylbenzyloxy)-3-(pyridin-3-ylamino)benzo[d]isoxazole (formula Ⅰ-1)-), 2-benzyloxy- Synthesis of 6-Fluorobenzonitrile (Compound 1)
[0047]
[0048] Dissolve 5.0g (36.5mmol, 1.0eq) of 2-cyano-3-fluorophenol, 10.0g (73mmol, 2.0eq) of potassium carbonate and 200mg of potassium iodide in 100mL of acetonitrile, and then add 6.55g (38.3mmol, 1.05eq) Benzyl bromide, after addition, reacted at room temperature for 12h, then distilled off most of the solvent under reduced pressure, added water, extracted with ethyl acetate, washed the organic phase with saturated brine, anhydrous Na 2 SO 4 Drying, concentration, using silica gel as a sample, and column chromatography (petroleum ether: ethyl acetate = 15:1) gave compound 4, 8.0 g of a white solid, with a yield of 96%.
[0049] After testing, the structure is correct, and the test results are as follows: MS (ESI) (m / z): 228.0 (M+H) + . 1 H NMR (400MHz, DMSO-d 6 )δ7.73–7.62(m,1H),7.43(d,J=7....
Embodiment 2
[0068] Embodiment 2: compound formula I-2, the synthesis of I-4~I-27
[0069]
[0070]
[0071]With reference to the conditions of the sixth step synthesis compound formula I-1 in the embodiment example 1, react with corresponding substituted benzyl bromide and 3-(pyridin-3-ylamino)-4-hydroxybenzo[d]isoxazole to obtain the corresponding Target compound formula I-2 and I-4 to I-27, specifically: 4-(2-chloro-5-fluorobenzyloxy)-3-(pyridin-3-ylamino)benzo[d]isox Azole (formula Ⅰ-2); 4-(2-fluorobenzyloxy)-3-(pyridin-3-ylamino)benzo[d]isoxazole (formula Ⅰ-4); 4-(3-fluoro Benzyloxy)-3-(pyridin-3-ylamino)benzo[d]isoxazole (formula Ⅰ-5); 4-(4-fluorobenzyloxy)-3-(pyridin-3-ylamino ) benzo[d] isoxazole (formula Ⅰ-6); 4-(2-chlorobenzyloxy) 3-(pyridin-3-ylamino) benzo[d] isoxazole (formula Ⅰ-7) ; 4-(3-chlorobenzyloxy)-3-(pyridin-3-ylamino)benzo[d]isoxazole (Formula Ⅰ-8); 4-(4-chlorobenzyloxy)-3- (Pyridin-3-ylamino)benzo[d]isoxazole (Formula Ⅰ-9); 4-(2-methylbenzyloxy)-3-(pyridin-3...
Embodiment 3
[0098] Example 3: Preparation of 3-(pyridin-3-ylamino)-4-(2-trifluoromethoxy-5-chlorobenzyloxy)-benzo[d]isoxazole (formula Ⅰ-3)
[0099] One), the synthesis of 2-trifluoromethoxy-5-chlorobenzaldehyde (compound 13)
[0100]
[0101]Dissolve 1.0g (5.1mmol, 1.0eq) of 4-trifluoromethoxychlorobenzene in 20mL of anhydrous tetrahydrofuran, under nitrogen protection, cool to -80°C, add dropwise 3.1mL (6.1mmol, 1.2eq) of 2M LDA, After 15 minutes of dripping, keep at -80°C for 20 minutes, add 0.47mL DMF, slowly raise the temperature to -50°C for 40 minutes, add 1.22g (20.4mmol, 4.0eq) acetic acid to quench the reaction, add water, extract with ethyl acetate, saturated saline Wash the organic phase with anhydrous Na 2 SO 4 Drying, concentration, silica gel as a sample, chromatographic column separation (petroleum ether: ethyl acetate = 20:1) gave compound 13, 800 mg of light yellow oil, yield 70%.
[0102] After testing, the structure is correct, and the test results are as follows...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com