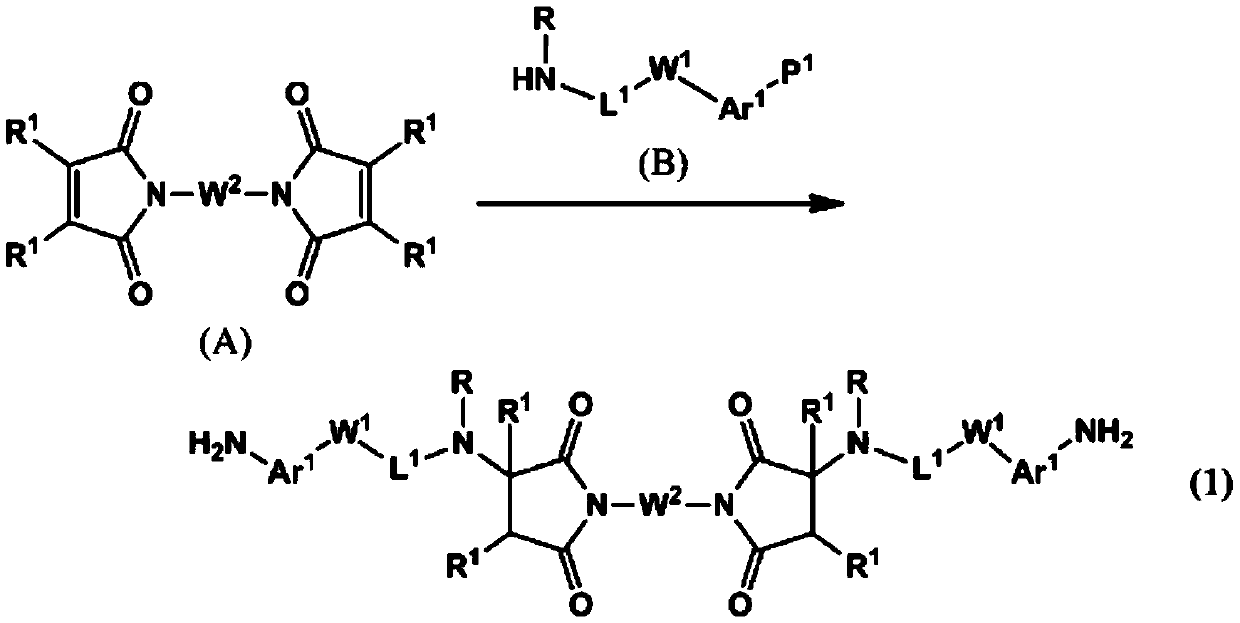
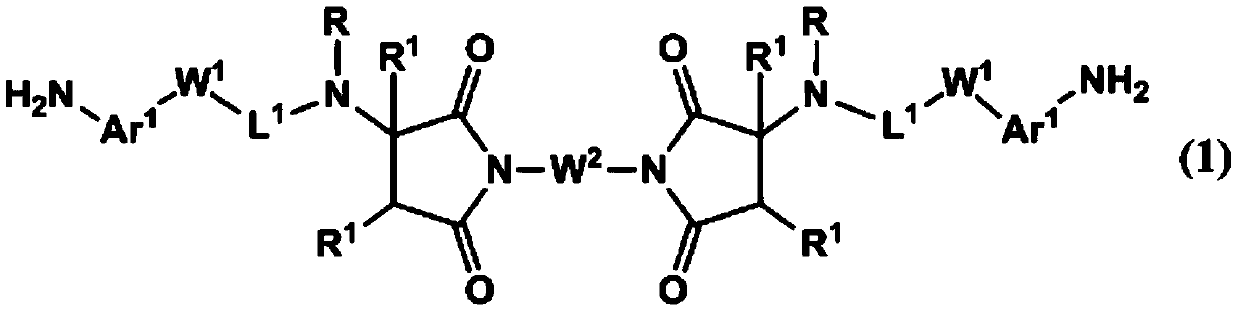
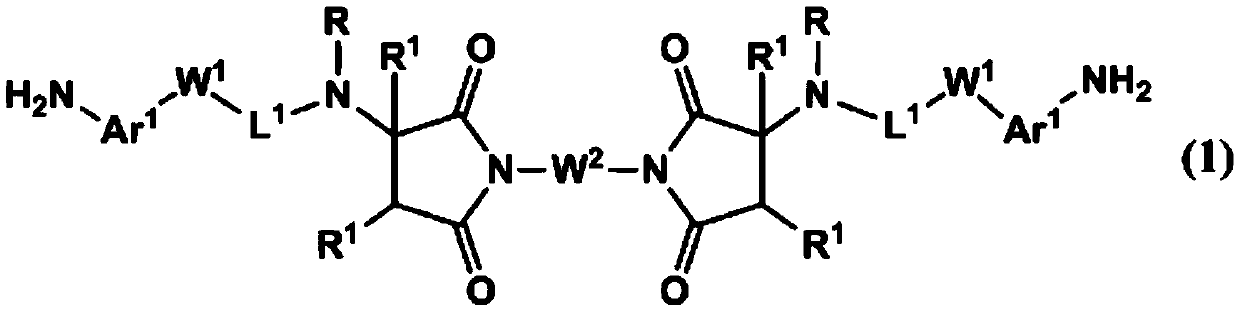
Novel polymer and diamine compound, liquid crystal alignment agent, liquid crystal alignment film, and liquid crystal display element
A liquid crystal aligning agent, amine compound technology, applied in optical elements, polarizing elements, organic chemistry, etc., can solve the problems of lower brightness, lower liquid crystal display quality, higher ion density, etc., and achieve high voltage retention and brush abrasion resistance. , indicates the effect of excellent characteristics and high availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0188] The present invention will be described in more detail with reference to examples below, but the present invention is not limited thereto. The abbreviations of the compounds used in the present examples and comparative examples and the method of property evaluation are as follows.
[0189] NMP: N-methyl-2-pyrrolidone
[0190] BCS: Butyl Cellosolve
[0191] GBL: gamma-butyrolactone
[0192] DA-1-1: a compound represented by the following formula DA-1-1
[0193] DA-1-2: a compound represented by the following formula DA-1-2
[0194] DA-1-3: a compound represented by the following formula DA-1-3
[0195] DA-1-4: a compound represented by the following formula DA-1-4
[0196] DA-1-5: a compound represented by the following formula DA-1-5
[0197] DA-1-6: a compound represented by the following formula DA-1-6
[0198] DA-1-7: a compound represented by the following formula DA-1-7
[0199] DA-1-8: a compound represented by the following formula DA-1-8
[0200] DA-1-9...
Embodiment A1
[0203] (Embodiment A1: synthesis of specific diamine)
[0204] Synthesis of (DA-1-1)
[0205]
[0206]After adding 8 g (29.8 mmol) of N,N'-(1,4-phenylene) bismaleimide and 160 g of tetrahydrofuran (henceforth THF) to a flask, it cooled with ice. To this mixture was added 9.801 g (65.2 mmol) of 4-(2-methylaminoethyl)aniline. Then, after gradually returning to room temperature, it was stirred at room temperature for 1 day. After confirming the completion of the reaction, THF was distilled off under reduced pressure. 140 g of methanol was added to the obtained residue, followed by stirring. The resulting precipitate was filtered. The filtrate was added to 140 g of methanol, stirred and filtered again to obtain crystals. The obtained crystals were dried at 50° C. to obtain 12.44 g of the target compound (DA-1-1) (yield 73%, HPLC area percentage: 99.1%).
[0207] 1H-NMR (CDCl3, δppm): 7.35 (s, 4H), 6.88 (d, 4H), 6.49 (d, 4H), 4.82 (brs, 4H), 4.19 (dd, 2H), 2.89-2.98 (m, 2...
Embodiment A2
[0208] (Embodiment A2: synthesis of specific diamine)
[0209] Synthesis of (DA-1-2)
[0210]
[0211] 1.00 g (3.73 mmol) of N,N'-(1,4-phenylene) bismaleimide and 10 g of THF were added to the flask, followed by cooling with ice. To this mixture was added 1.00 g (8.19 mmol) of 3-aminomethylaniline. Then, after gradually returning to room temperature, it was stirred at room temperature for 1 day. After confirming the completion of the reaction, THF was distilled off under reduced pressure. Isopropanol (hereinafter referred to as IPA) was added to the obtained residue and stirred to obtain crystals. The obtained crystals were filtered, and the filtered crystals were dried at 50° C. to obtain 0.55 g of the target compound (DA-1-2) (yield 29%).
[0212] 1H-NMR (d6-DMSO, δppm): 7.40(s, 4H), 6.93-6.99(m, 2H), 6.58-6.61(m, 2H), 6.49-6.53(m, 2H), 6.42-6.47(m , 2H), 4.99(brs, 4H), 3.71-3.90(m, 6H), 3.00-3.08(m, 2H), 2.87(brs, 2H), 2.56-2.63(m, 2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com