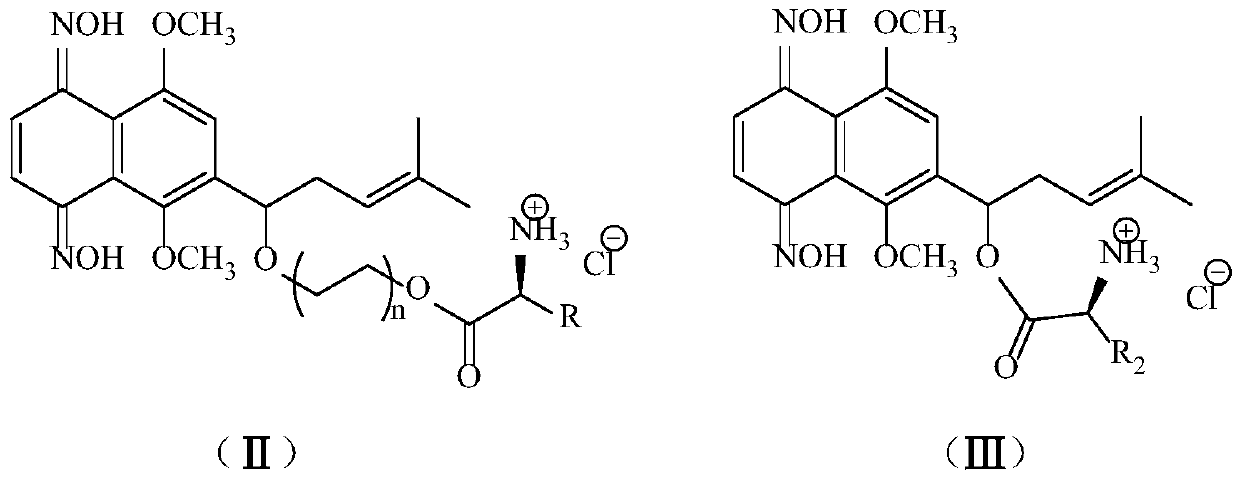
Racemate alkannin oxime amino acid ester derivatives and preparation method and applications thereof
A technology of racemate and amino acid ester, applied in the field of medicine, can solve the problems of disappearance of anti-tumor activity and reduced activity of target substances, and achieve the effects of simple preparation method, improved permeability and good prospect of new drug development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
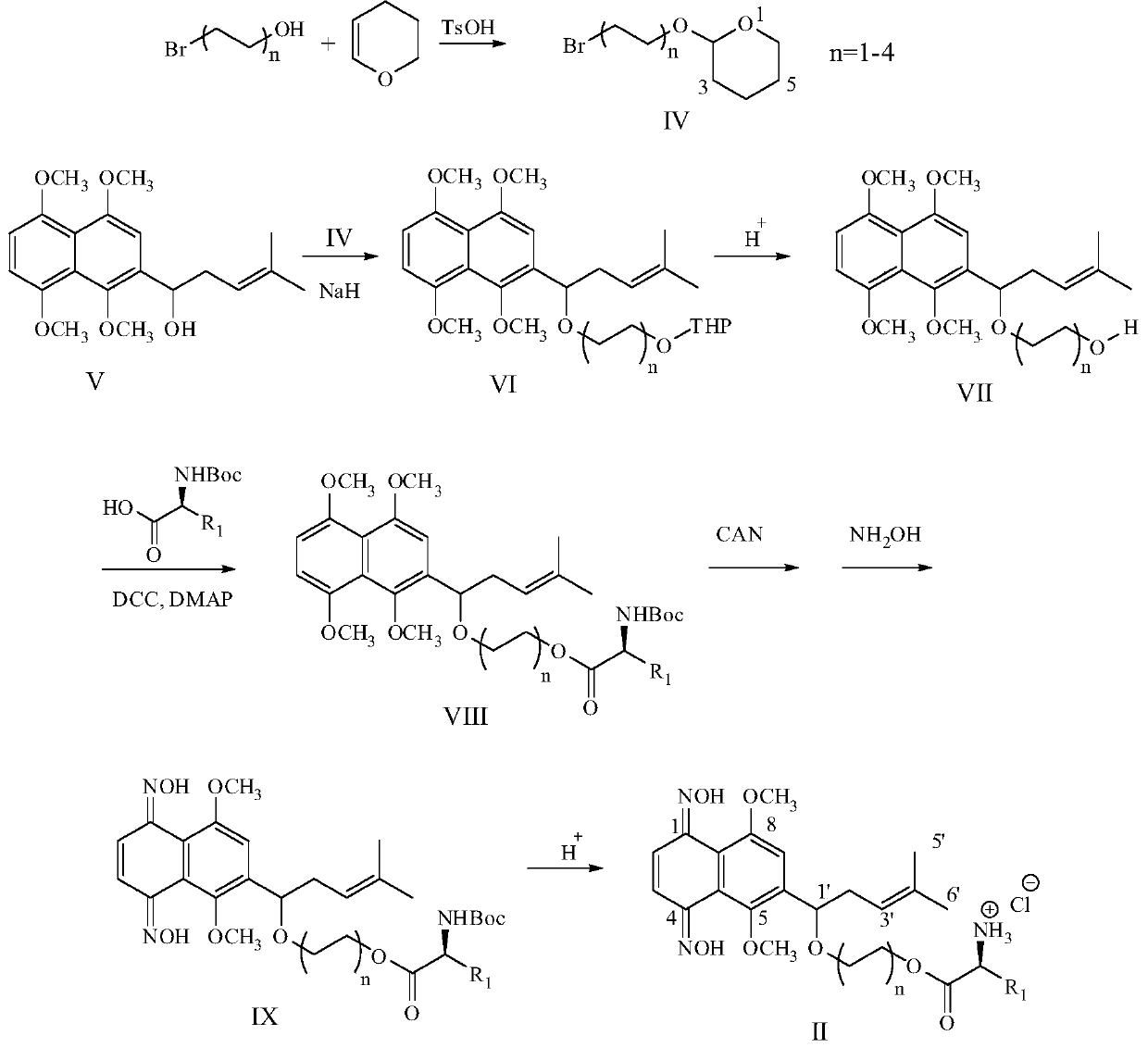
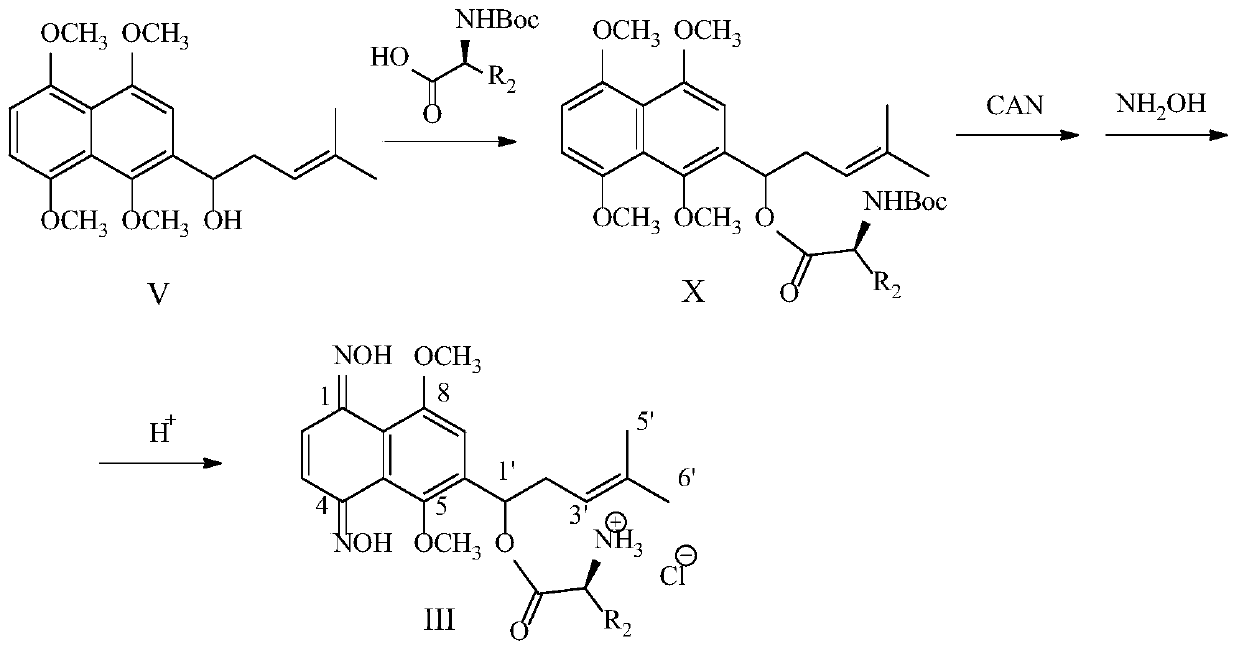
[0041]This embodiment relates to a kind of 2-[1-(2-hydroxyethoxy)-4-methyl-3-pentenyl]-1,4,5,8-tetramethoxy with structural formula (VII) The preparation method of naphthalene (VII-1, n=1), such as figure 1 shown, including the following steps:
[0042] Step 1: Dissolve 2-bromoethanol in anhydrous dichloromethane, add a catalytic amount of p-toluenesulfonic acid or pyridine p-toluenesulfonic acid, then add 1.5 times the equivalent of 3,4-dihydro-2H-pyran , Stir at room temperature for 5h. The reaction was quenched by adding saturated aqueous sodium bicarbonate, CH 2 Cl 2 Extract and combine the organic layers. The organic layer was washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, concentrated, and subjected to silica gel column chromatography to obtain 2-(2-bromoethoxy)-tetrahydropyran (IV-1, n=1) . The product was a colorless oil with a yield of 75%. 1 H NMR (300MHz, CDCl 3 ):δ4.67(m,1H),4.01(dt,J=12.3,6.3Hz,1H),3.88(m,1H),3.76(...
Embodiment 2
[0045] This embodiment relates to a kind of 2-[1-(4-hydroxybutoxy)-4-methyl-3-pentenyl]-1,4,5,8-tetramethoxy with structural formula (VII) The preparation method of naphthalene (VII-2, n=2), such as figure 1 shown, including the following steps:
[0046] The steps of this example are the same as the steps of Example 1, in Step 1, 2-bromoethanol is replaced by 4-bromo-1-butanol. Product C was in the form of a light brown oil, with a yield of 82%. 1 H NMR (300MHz, CDCl 3 ):δ6.91(s,1H,H-3),6.75(s,2H,H-6,7),5.18(t,1H,H-3'),4.90(t,1H,H-1' ),3.90(s,6H,ArOCH 3 ),3.84(s,3H,ArOCH 3 ),3.66(s,3H,ArOCH 3 ),3.51(t,2H,CH 2 OH),3.21(2H,m,OCH 2 ),2.42(2H,m,H-2'),1.59(3H,s,CH 3 ),1.46(3H,s,CH 3 ),1.20-1.40(4H,m,CH 2 OH).
Embodiment 3
[0048] This embodiment relates to a kind of 2-[1-(6-hydroxyhexyloxy)-4-methyl-3-pentenyl]-1,4,5,8-tetramethoxy with structural formula (VII) The preparation method of naphthalene (VII-3, n=3), such as figure 1 shown, including the following steps:
[0049] The steps of this example are the same as the steps of Example 1, in Step 1, 2-bromoethanol is replaced by 6-bromo-1-hexanol. The product C was in the form of a light brown oil with a yield of 75%. 1 H NMR (300MHz, CDCl 3 ):δ6.95(s,1H,H-3),6.81(2H,s,H-6,7),5.22(1H,t,H-3'),4.96(1H,t,H-1' ),3.92(6H,s,ArOCH 3 ),3.82(3H,s,ArOCH 3 ),3.72(3H,s,ArOCH 3 ),3.47(2H,t,CH 2 OH),3.25(2H,m,OCH 2 ),2.45(2H,m,H-2'),1.64(3H,s,CH 3 ),1.52(3H,s,CH 3 ),1.20-1.40(8H,m,CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com