Recombinant protein for weight loss, vaccine and construction method thereof
A technology of recombinant protein and construction method, applied in the field of vaccines, can solve the problems of drug accumulation, central side effects, accumulation, etc., achieve the effect of increasing secretion, reducing body weight and fat content, and solving long-term use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0100] Example 1-------Construction and expression of recombinant protein 1, including:
[0101] 1) Recombinant protein 1 protein sequence>Protein1LIJUN_20180822:
[0102] MAPQTITELCSEYRNTQIYTINDKILSYTESMAGKREMVIITFKSGETFQVEVPGSQHIDSQKKAIERMKDTLRITYLTETKIDKLCVWNNKTPNSIAAISMKNEFAKFVAAWTLKAAAGSAGCKNFFWKTFTSCLEHHHHHH.
[0103] The first position of the N-terminal is methionine, which is because the expression system of Escherichia coli is used. The 2nd to 104th positions (103AA) of the N-terminus are LTB (Escherichia coli heat-labile enterotoxin B subunit). The 105th-106th position of the N-terminal is the linker sequence EF, the 107-119th position of the N-terminal is the immune enhancement sequence, the 120-121st position of the N-terminal is the linker sequence GS, the 122-135th position is the somatostatin sequence, and the 136th- The 137th position is the linker sequence LE, and the 138-143rd position is the 6His tag.
[0104] Recombinant protein 1 protein gene sequence>P...
example 3
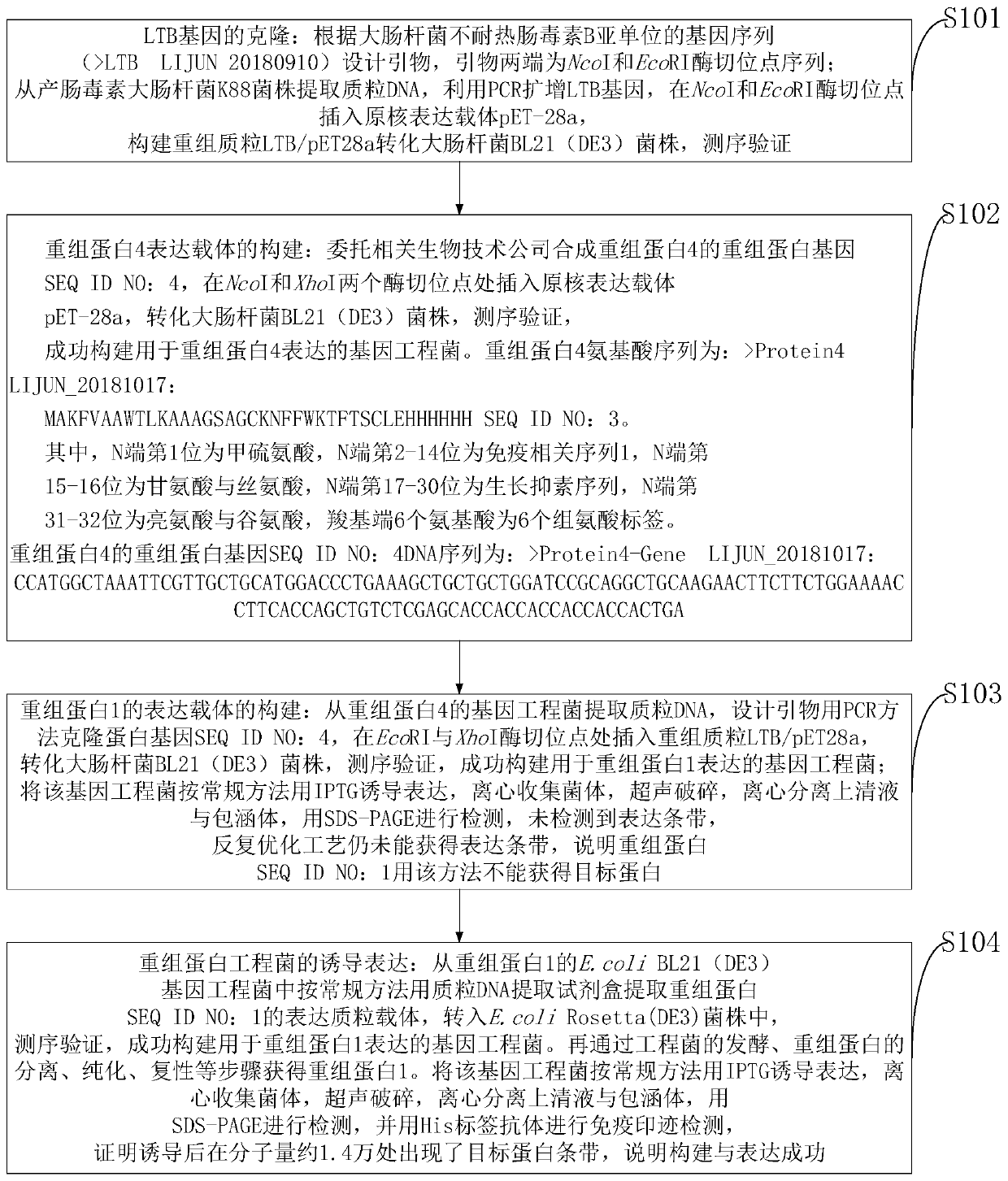
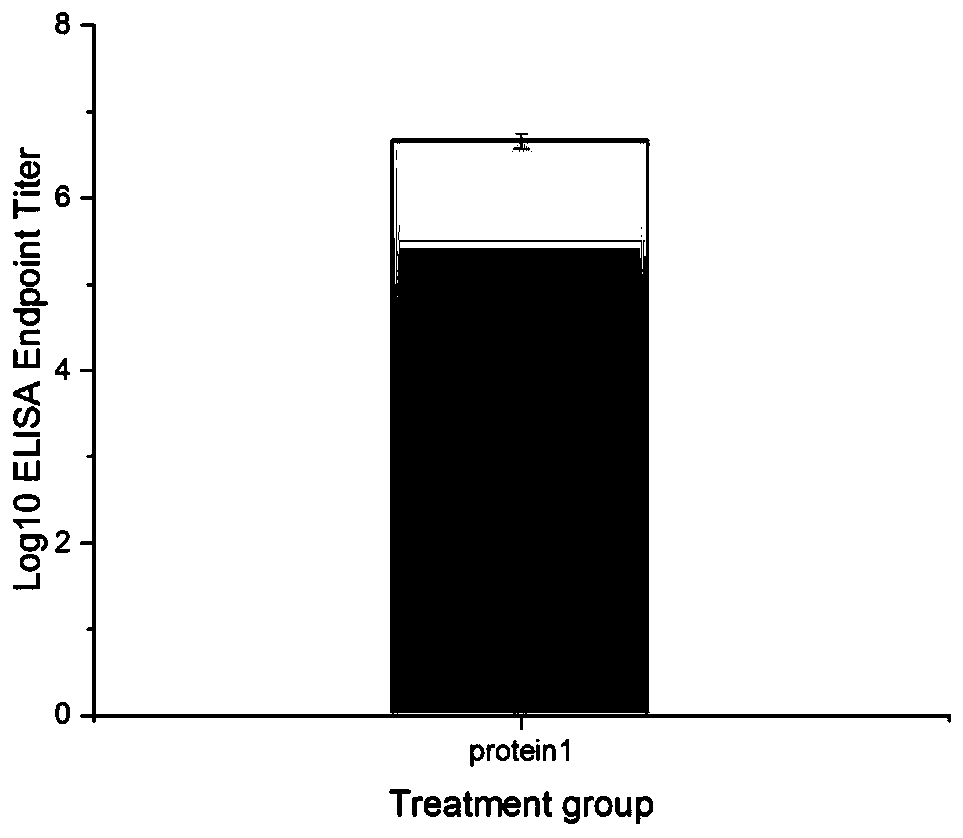
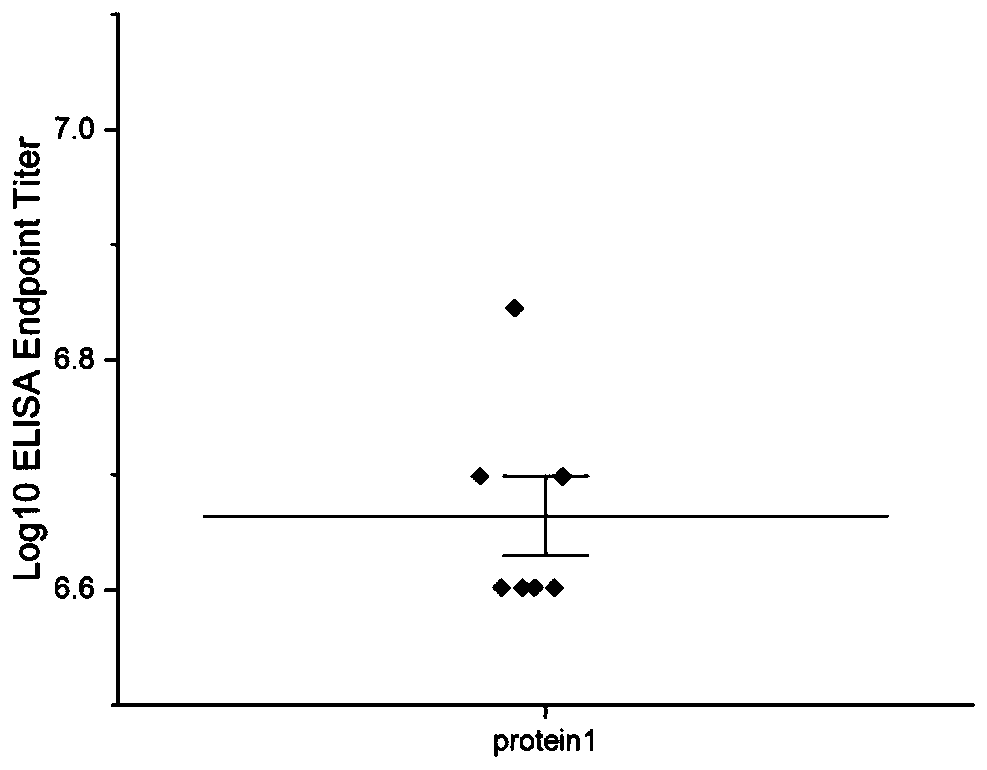
[0120] Example 3-------The immunogenicity of recombinant protein 1, including:
[0121] Immunization: 16 6-week-old Kunming mice (SPF grade, female) were randomly divided into experimental group and control group, with 8 mice in each group, and footpad immunization was carried out on day 0, 21, and 42 respectively. The recombinant protein was used as the immunogen, and the immunization dose was 50 μg per mouse, and the control group was immunized with PBS buffer. One week after the last immunization, blood was collected from the orbital venous plexus of the mice, and the serum was separated and stored at -20°C.
[0122] Tissue section: After blood collection, all mice were killed, dissected, immune-related organs were separated, weighed, fixed with 4% paraformaldehyde, and sent to the company to make pathological sections.
[0123] Western blot: take the purified recombinant protein for SDS-PAGE electrophoresis, cut off the gel containing the target protein after electrophore...
example 4
[0125] Example 4-------Construction and expression of recombinant protein 2, including:
[0126] 1) Recombinant protein 2 protein sequence>Protein2LIJUN_20180822:
[0127] MAPQTITELCSEYRNTQIYTINDKILSYTESMAGKREMVIITFKSGETFQVEVPGSQHIDSQKKAIERMKDTLRITYLTETKIDKLCVWNNKTPNSIAAISMKNEFQYIKANSKFIGITEGSAGCKNFFWKTFTSCLEHHHHHH.
[0128] The first position of the N-terminal is methionine, which is because the expression system of Escherichia coli is used. The 2nd to 104th positions (103AA) of the N-terminus are LTB (Escherichia coli heat-labile enterotoxin B subunit). The 105th-106th position of the N-terminal is the linker sequence EF, the 107-120th position of the N-terminal is the immune enhancement sequence, the 121-122nd position of the N-terminal is the linker sequence GS, the 123-136th position is the somatostatin sequence, and the 137th- The 138th position is the linker sequence LE, and the 139th-144th position is the 6His tag.
[0129] Recombinant protein 2 gene sequence>Protei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com