Preparation method and anti-asthma application of levo(-)terbutaline
A terbutaline and reaction technology, which is applied in the field of chemical resolution of β2 receptor agonists, can solve problems such as difficulty in chiral resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
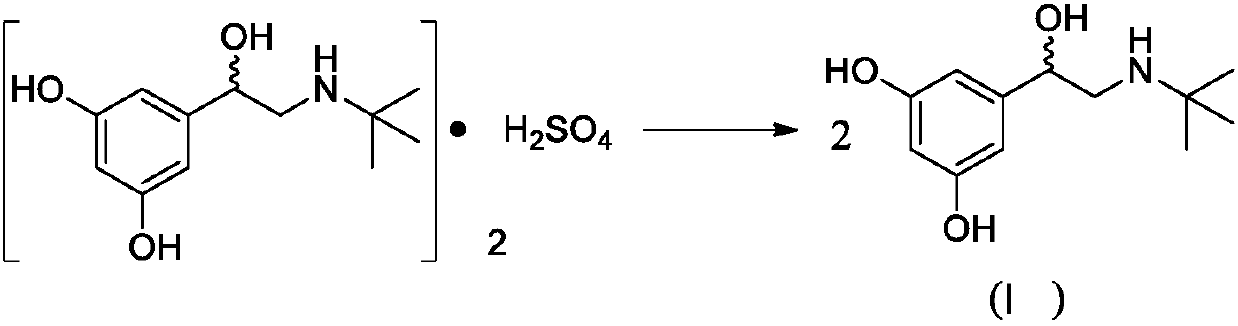
[0042] Dissolve racemic terbutaline sulfate (5.0g, 9.12mmol) and anhydrous potassium carbonate (1.3g, 9.42mmol) in 31.5ml methanol, reflux and react at 70°C for about 2h. After the reaction is complete, stop heating and stir. Cool to room temperature, filter with suction, and collect the filtrate.
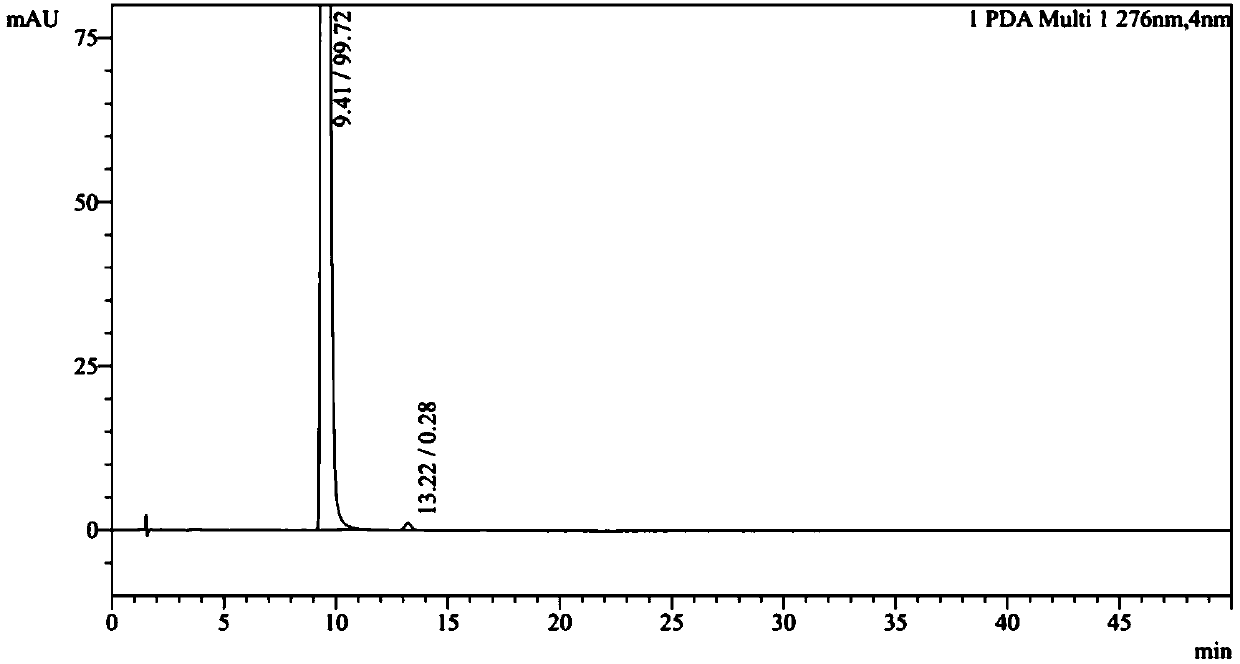
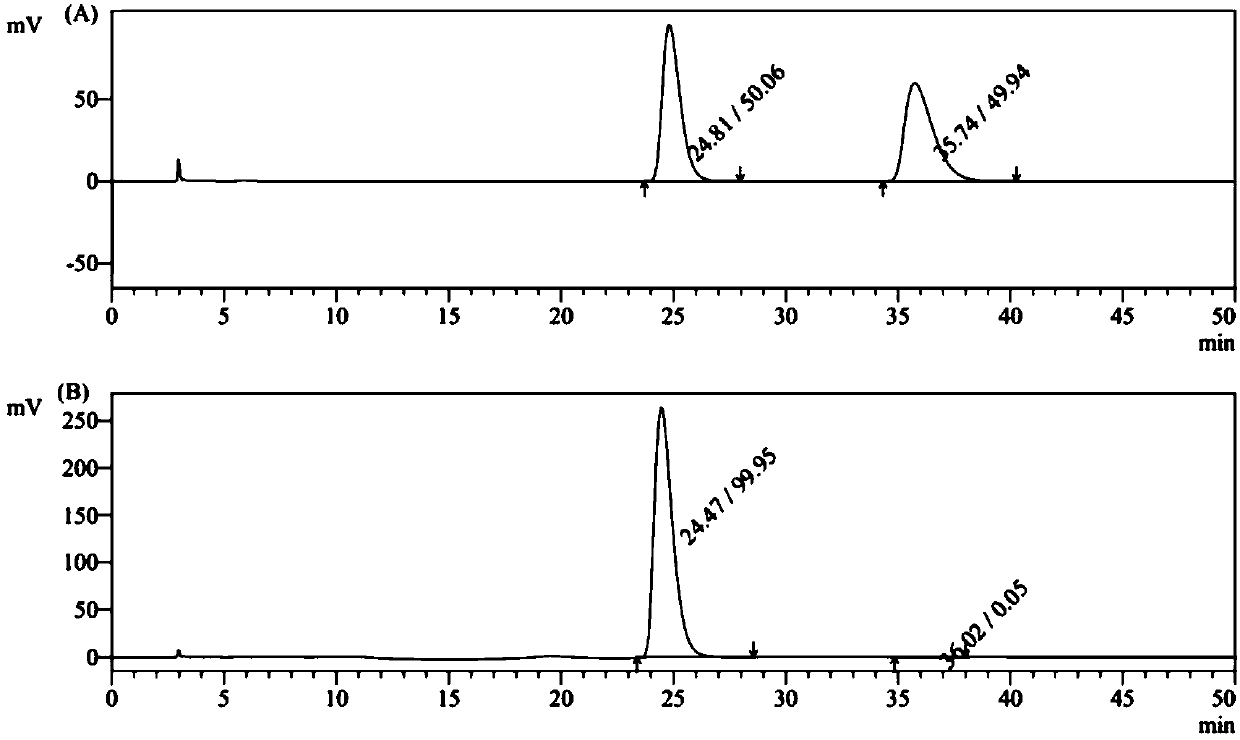
[0043] D-(+)-DTTA (7.0g, 18.1mmol) was added to the filtrate, placed at 80°C for recrystallization, added with dichloromethane about 10ml, refluxed for 2h, allowed to stand at room temperature for 24h to crystallize, and filtered to obtain levorotatory ( -) Crude terbutaline tartrate, repeat this operation three times to obtain about 2.6 g of L-(-) terbutaline tartrate, the optical purity of which is 99.8%, and the yield is 47%.
[0044] Take 2g of L-(-)terbutaline tartrate and add 10% K 2 CO 3 The solution is about 10ml, extracted four times with 30ml ethyl acetate, combined the organic phases, dried over anhydrous magnesium sulfate, spin-dried the solvent, dissolve the solid powder wi...
Embodiment 2
[0046] Dissolve racemic terbutaline sulfate (5.0g, 9.12mmol) and anhydrous potassium carbonate (1.3g, 9.42mmol) in 31.5ml methanol, reflux and react at 70°C for about 2h. After the reaction is complete, stop heating and stir. Cool to room temperature, filter with suction, and collect the filtrate.
[0047] D-(+)-DTTA (7.0g, 18.1mmol) was added to the filtrate, placed at 80°C for recrystallization, added with dichloromethane about 15ml, refluxed for 2h, allowed to stand at room temperature for 24h to crystallize, and filtered to obtain levorotatory ( -) Crude terbutaline tartrate. Repeat this operation three times to obtain about 2.7 g of L-(-) terbutaline tartrate. The optical purity is 99.8% and the yield is 47%.
[0048] Dissolve 2g of L-(-)terbutaline tartrate in 10ml of water, add about 1ml of ammonia solution, extract four times with 40ml of ethyl acetate, combine the organic phases, dry with anhydrous magnesium sulfate, spin dry the solvent, and use the solid powder Dissolve...
Embodiment 3
[0050] Dissolve racemic terbutaline sulfate (10.0g, 18.24mmol) and anhydrous potassium carbonate (2.6g, 18.84mmol) in 63ml methanol, reflux for about 2h at 70°C, stop heating, stir and cool To room temperature, suction filtration, and collect the filtrate.
[0051] D-(+)-DTTA (14.0g, 36.2mmol) was added to the filtrate, placed at 80°C for recrystallization, added with dichloromethane about 30ml, refluxed for 2h, allowed to stand at room temperature for 24h to crystallize, and filtered to obtain levorotatory ( -) Crude terbutaline tartrate. Repeat this operation three times to obtain about 5.6 g of L-(-) terbutaline tartrate. The optical purity is 99.8% and the yield is 50%.
[0052] Take 2g of L-(-) terbutaline tartrate and add saturated K 2 CO 3 The solution solution is about 10ml, extracted four times with 40ml of isopropanol: dichloromethane (1:3), the organic phases are combined, dried over anhydrous magnesium sulfate, spin-dried to dry the solvent, and the solid powder is diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com