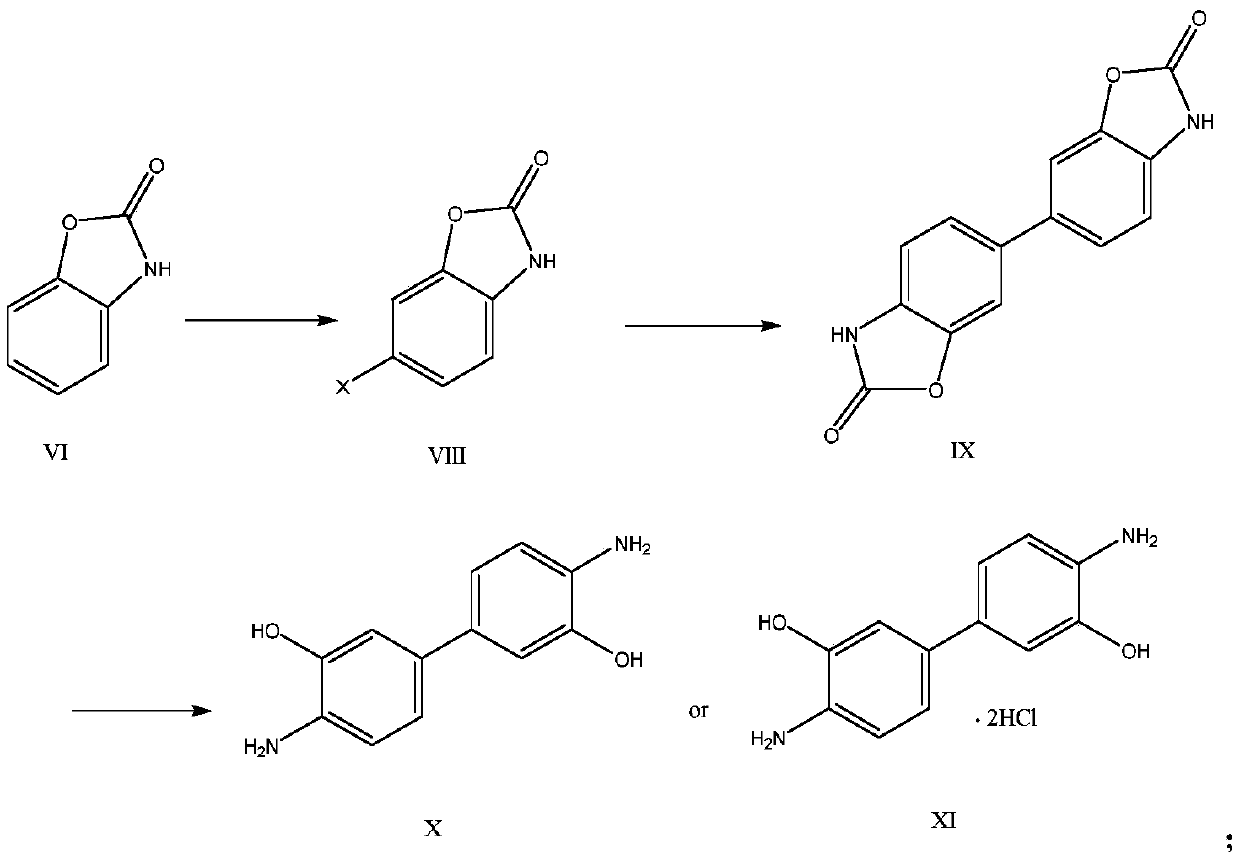
Method for synthesizing benzoxazole compound by using nitration by-products of aromatic hydrocarbon and application of benzoxazole compound
A nitration technology of benzoxazole and aromatic hydrocarbons, which is applied in the chemical industry, can solve the problems of difficult use, low quality of sulfur black dyes, and increase economic benefits, and achieve the effects of increasing economic benefits of enterprises, expanding the scope of exhibits of enterprises, and high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
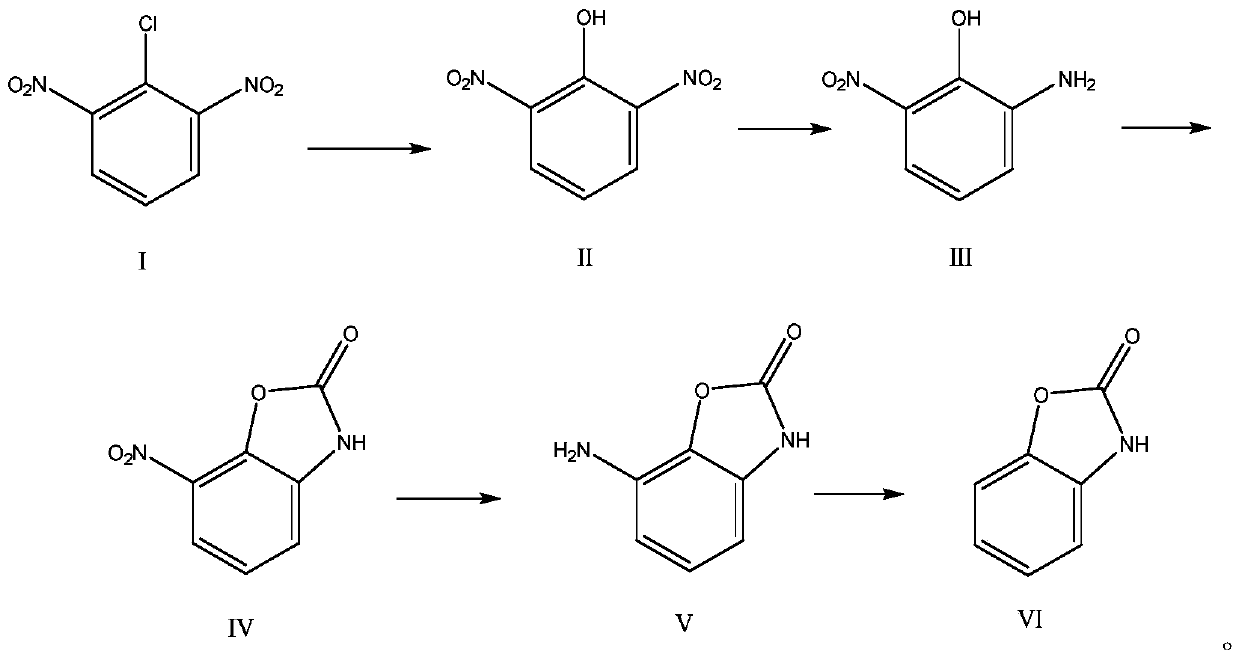
[0043] The synthesis of embodiment 1.2-benzoxazolone (VI)
[0044] 1) Synthesis of 2,6-dinitrophenol (II)
[0045] Mix 2945.6kg of aromatic hydrocarbon nitration by-products (a mixture of 2,4-dinitrochlorobenzene and 2,6-dinitrochlorobenzene (I)), 5891.3kg of water, and 3879.1kg of liquid caustic soda (30%), Stir at 50-85°C until the reaction is complete, add 14728kg of 1,2-dichloroethane, acidify the system with hydrochloric acid until the pH of the system is 1, and separate layers to obtain 2,6-dinitrophenol (II)-1, 2-dichloroethane solution.
[0046] 2) Synthesis of 2-amino-6-nitrophenol (III)
[0047] Mix the 2,6-dinitrophenol (II)-1,2-dichloroethane solution obtained in step 1) and 15kg of palladium carbon, evacuate the nitrogen, stir, and pass it into the 0.2-0.6MPa hydrogen gas, stirred to the end of the reaction; the catalyst was recovered by filtration, and allowed to stand for stratification to obtain 2-amino-6-nitrophenol (III)-dichloroethane solution.
[0048] ...
Embodiment 2
[0054] The synthesis of embodiment 2.2-benzoxazolone (VI)
[0055] 1) Preparation of the mixture (I) of 2,6-dinitrobenzene chloride
[0056] The mixture of 1000kg2,4-dinitrochlorobenzene and 2,6-dinitrochlorobenzene (I) is dropped into the fractional crystallization separator, and through the fractional crystallization process, 83.1kg of 2 with a purity of 99.3% are obtained. , 4-dinitrochlorobenzene simple compound, 742.4kg purity is 99.5% 2,6-dinitrochlorobenzene (I) simple compound and 174.5kg2,4-dinitrochlorobenzene and 2,6 - mixtures of dinitrochlorobenzene (I);
[0057] Among them, 83.1kg of 2,4-dinitrochlorobenzene with a purity of 99.3% is sold as the main product produced by the enterprise;
[0058] The mixture of 174.5 kg of 2,4-dinitrochlorobenzene and 2,6-dinitrochlorobenzene (I) was returned to the next batch of fractional crystallization process.
[0059] 2) Synthesis of 2-benzoxazolone (VI)
[0060] The 742.4kg purity that obtains with above-mentioned proces...
Embodiment 3
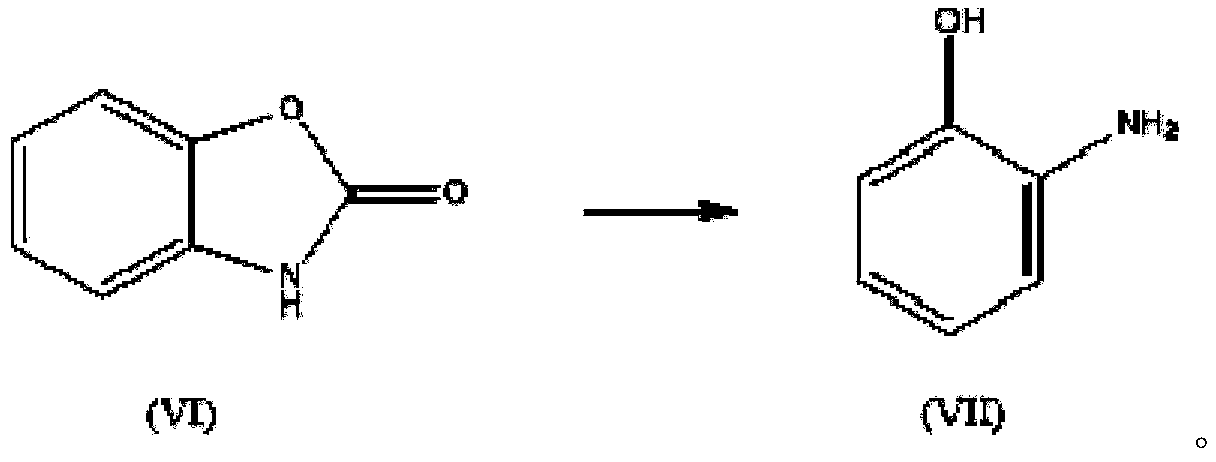
[0062] Embodiment 3. the synthesis of o-aminophenol (VII)
[0063] 135.1kg of 2-benzoxazolone (VI) with a purity of 99.3% obtained through the process of Example 1 was mixed with 533.3kg of liquid caustic soda (15%), stirred, heated to 95-105°C, and the pressure was 0-0.15MPa , until the reaction was complete, hydrochloric acid was acidified until the pH of the system was 7, and solid-liquid separation was carried out to obtain 100.7 kg of o-aminophenol (VII) with a purity of 99.0%, with a yield of 96.5%; the residue in the filtrate could be extracted and recovered with dichloroethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com