Ionic liquid-promoted one-pot method for synthesizing 4H-pyranocoumarin derivatives
A technology of coumarin derivatives and ionic liquids, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., to achieve convenient recycling, high atom economy, and easy operation and the effect of simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
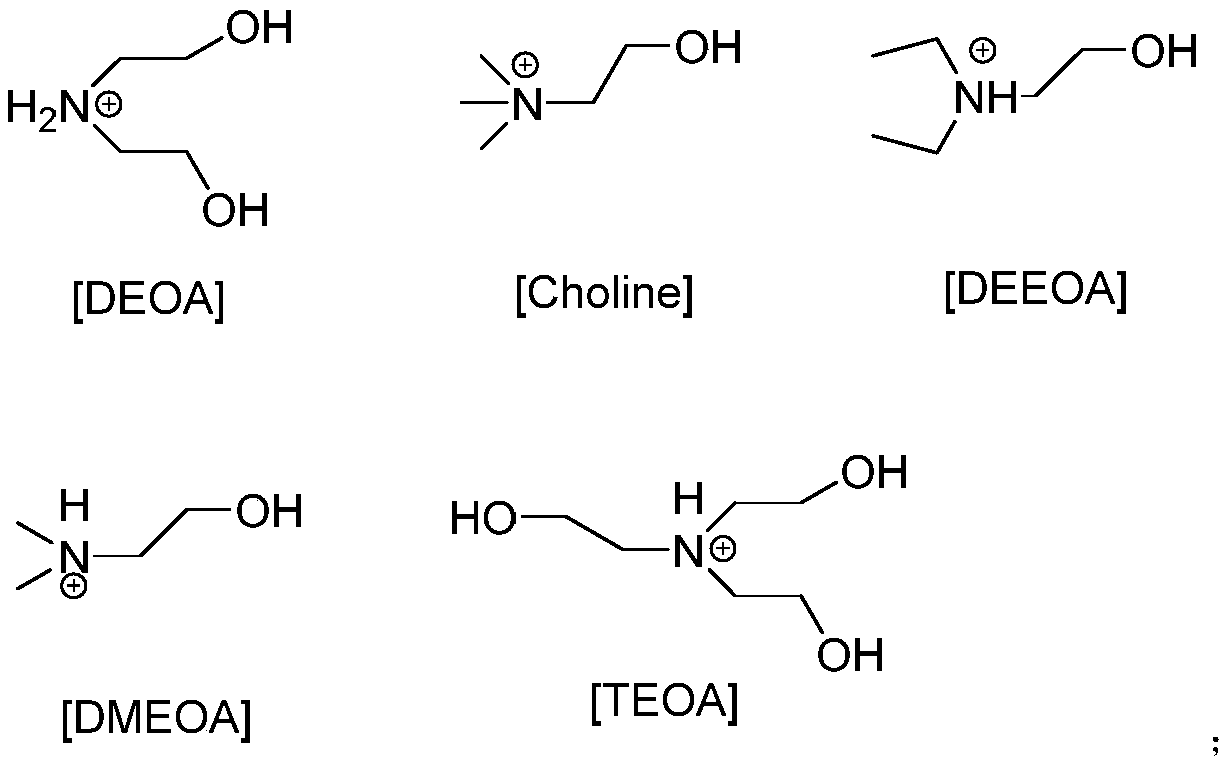
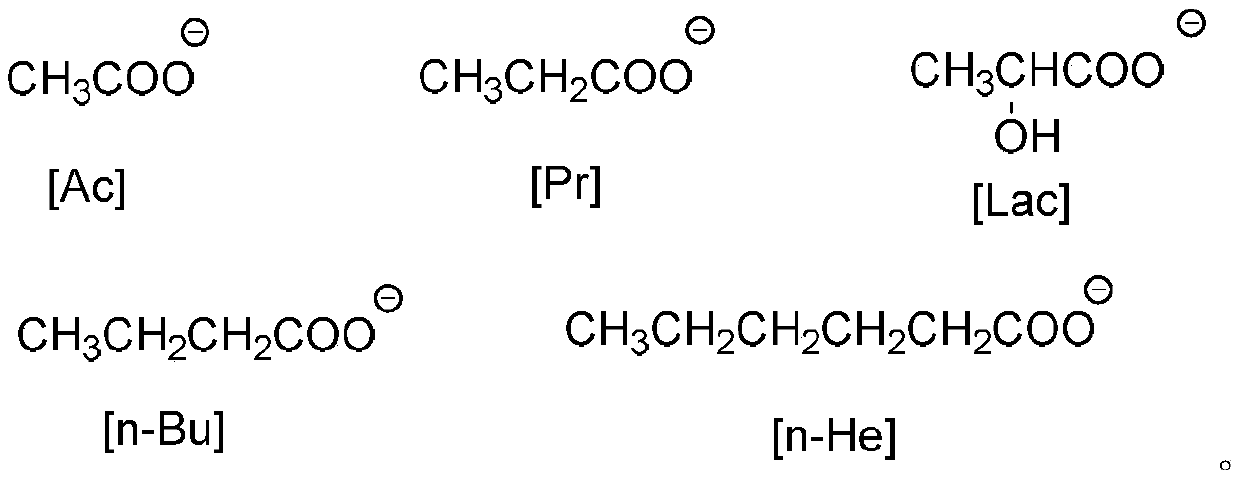
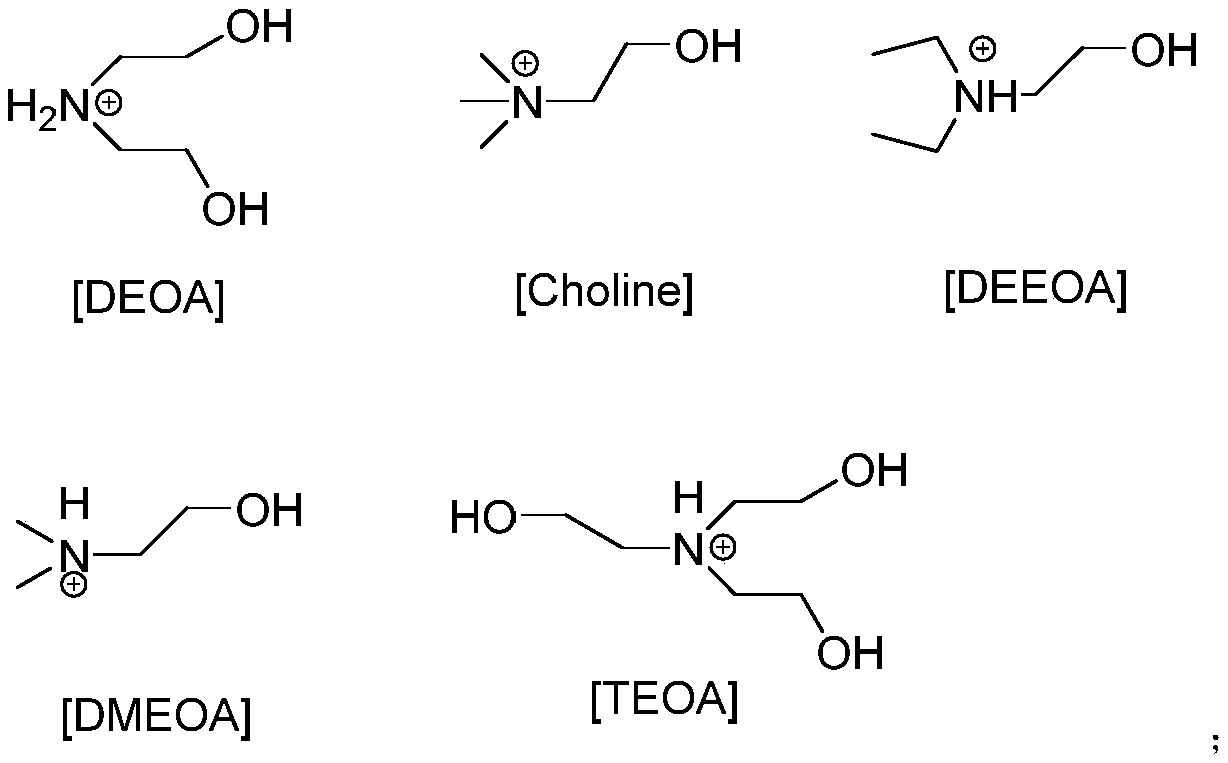
[0022] Add 0.5mmol functionalized ionic liquid [TEOA][Ac], 0.5mmol 4-hydroxycoumarin, 0.5mmol benzaldehyde and 0.5mmol malononitrile in a round bottom flask, stir and mix with magnetic force, and stir at room temperature Reaction for 1.5h. TLC is used to track and monitor the whole process. After the reaction is over, add water twice for washing and centrifugation. After several times, wash with n-hexane to obtain pure product. Then it was placed in an oven and dried at 50°C, and the yield was 71%.
Embodiment 2
[0024] Add 0.5mmol functionalized ionic liquid [TEOA][Ac], 0.5mmol 4-hydroxycoumarin, 0.5mmol 4-nitrobenzaldehyde and 0.5mmol malononitrile into the round-bottom flask. The reaction was stirred at room temperature for 0.5h, and the whole process was monitored by TLC. After the reaction is over, add water twice for washing and centrifugation. After several times, wash with n-hexane to obtain pure product. Then it was placed in an oven and dried at 50°C, and the yield was 95%.
Embodiment 3
[0026] Add 0.5mmol functionalized ionic liquid [TEOA][Ac], 0.5mmol 4-hydroxycoumarin, 0.5mmol 3-nitrobenzaldehyde and 0.5mmol malononitrile into the round-bottom flask successively. The reaction was stirred at room temperature for 50 min. TLC is used to track and monitor the whole process. After the reaction is over, add water twice for washing and centrifugation. After several times, wash with n-hexane to obtain pure product. Then it was placed in an oven and dried at 50°C. The yield was 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com