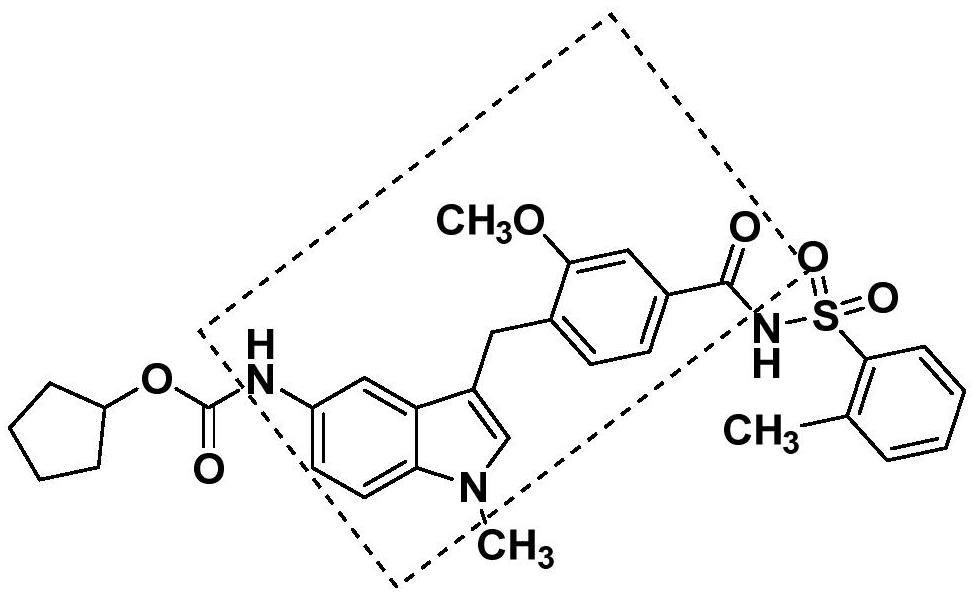
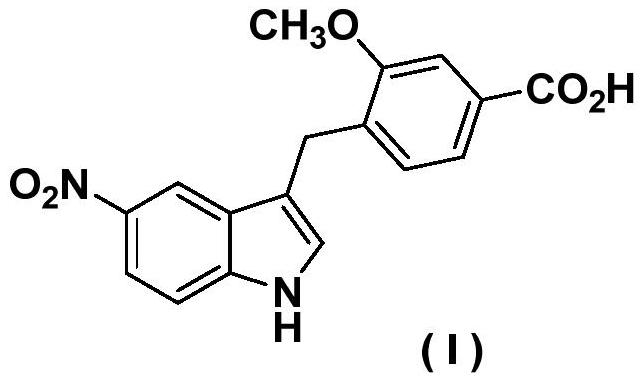
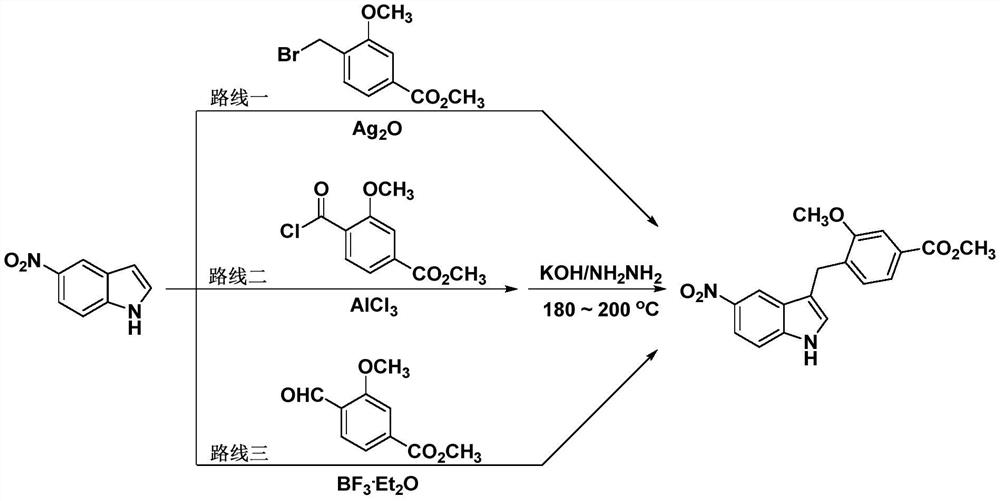
A kind of preparation technology of zafirlukast intermediate
A preparation process and a technology for intermediates, which are applied in the field of preparation processes for the intermediates of zafirlukast, a drug for treating asthma, can solve the problems of increased impurities, difficult purification, expensive raw materials/reagents, etc. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of methyl (5-nitro-1-toluenesulfonyl-1H-indol-3-yl)acetate:
[0025]
[0026] In a 250mL round-bottomed flask, add 5-nitroindole-3-carbaldehyde (19g, 0.1mol) and dissolve it in 150mL of dry dichloromethane, then add p-toluenesulfonyl chloride (19.1g, 0.1mol) and carbonic acid in sequence Potassium (27.6 g, 0.2 mol) was heated to reflux for 12 hours. TLC measured the disappearance of the raw materials, cooled to room temperature, allowed to stand, filtered with suction, and distilled the filtrate under reduced pressure to remove the organic solvent to obtain 29 g of a yellow solid. In a 250 mL round-bottomed flask, the above-mentioned 29 g of the yellow solid was dissolved in 100 mL of ethanol, and sodium borohydride (6.4 g, 0.17 mol) was added in batches with stirring, and the reaction was carried out at room temperature for 6 hours. TLC determined the disappearance of the raw materials, 1M aqueous hydrochloric acid was adjusted to pH 7, extracted with e...
Embodiment 2
[0036] Preparation of methyl 3-methoxy-4-[(5-nitro-1-toluenesulfonyl-1H-indol-3-yl)methyl]benzoate:
[0037] In a 250 mL round bottom flask, add methyl (5-nitro-1-toluenesulfonyl-1H-indol-3-yl)acetate (9.7 g, 0.025 mol) and methyl 3-methoxybenzoate ( 4.2 g, 0.025 mol) was dissolved in 50 mL of chloroform, acidified montmorillonite (8.4 g) was added under stirring, and the mixture was heated to 45° C. for reflux reaction for 4 hours. TLC measured the disappearance of the raw materials, cooled to room temperature, left standing, suction filtered, the filtrate was distilled under reduced pressure to remove the organic solvent to obtain a yellow solid, which was recrystallized from ethyl acetate / cyclohexane to obtain a white solid 3-methoxy-4-[(5 -Nitro-1-toluenesulfonyl-1H-indol-3-yl)methyl]benzoic acid methyl ester (8.1 g, 66% yield).
Embodiment 3
[0039] Preparation of methyl 3-methoxy-4-[(5-nitro-1-toluenesulfonyl-1H-indol-3-yl)methyl]benzoate:
[0040] In a 250 mL round bottom flask, add methyl (5-nitro-1-toluenesulfonyl-1H-indol-3-yl)acetate (9.7 g, 0.025 mol) and methyl 3-methoxybenzoate ( 4.2 g, 0.025 mol) was dissolved in 50 mL of 1,2-dichloroethane, acidified montmorillonite (8.4 g) was added under stirring, and the mixture was heated to 45° C. for reflux reaction for 4 hours. TLC measured the disappearance of the raw materials, cooled to room temperature, left standing, suction filtered, the filtrate was distilled under reduced pressure to remove the organic solvent to obtain a yellow solid, which was recrystallized from ethyl acetate / cyclohexane to obtain a white solid 3-methoxy-4-[(5 -Nitro-1-toluenesulfonyl-1H-indol-3-yl)methyl]benzoic acid methyl ester (7.8 g, 63% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com