Method of preparing formononetin ultrafine particles by supercritical antisolvent crystallization
A supercritical anti-solvent and formononetin technology, which is applied in the direction of anti-toxic agents, anti-inflammatory agents, and medical preparations containing active ingredients, can solve problems such as formononetin ultrafine particles that have not yet been seen, and achieve the improvement of biological Effects of utilization, enhanced adsorption, and increased specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: single factor experiment
[0043] Single factor experiment: the effect of injection flow rate on the particle size of formononetin
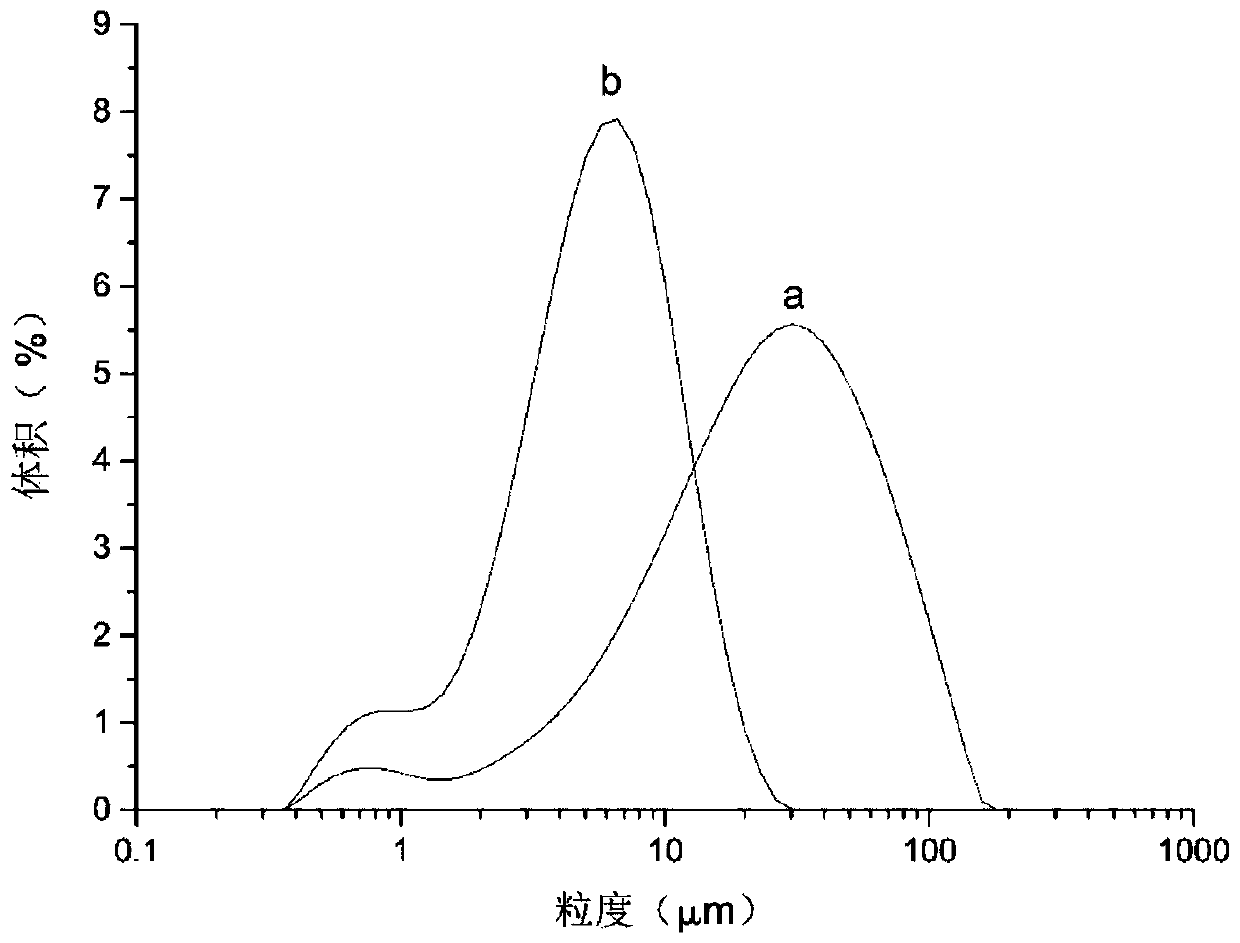
[0044] At crystallization temperature 42°C, crystallization pressure 10MPa, CO 2 Under the condition that the flow rate is 4~4.5L / min and the mass concentration of formononetin is 5mg / mL, the effect of the injection flow rate of 1.0, 1.4, 1.8, 2.2, 2.6mL / min on the volume average of formononetin particles was investigated. Influenced by the particle size, the volume average particle sizes were 9.185, 7.860, 6.146, 6.910, and 7.785 μm, so the injection flow rate of the optimal group was determined to be 1.8 mL / min.
[0045] Single Factor Experiment: Effect of Crystallization Pressure on Formononetin Particle Size
[0046] At a crystallization temperature of 42°C, an injection flow rate of 1.8mL / min, CO 2 Under the conditions of flow rate of 4-4.5L / min and formononetin mass concentration of 5mg / mL, the effects of crystalliza...
Embodiment 2
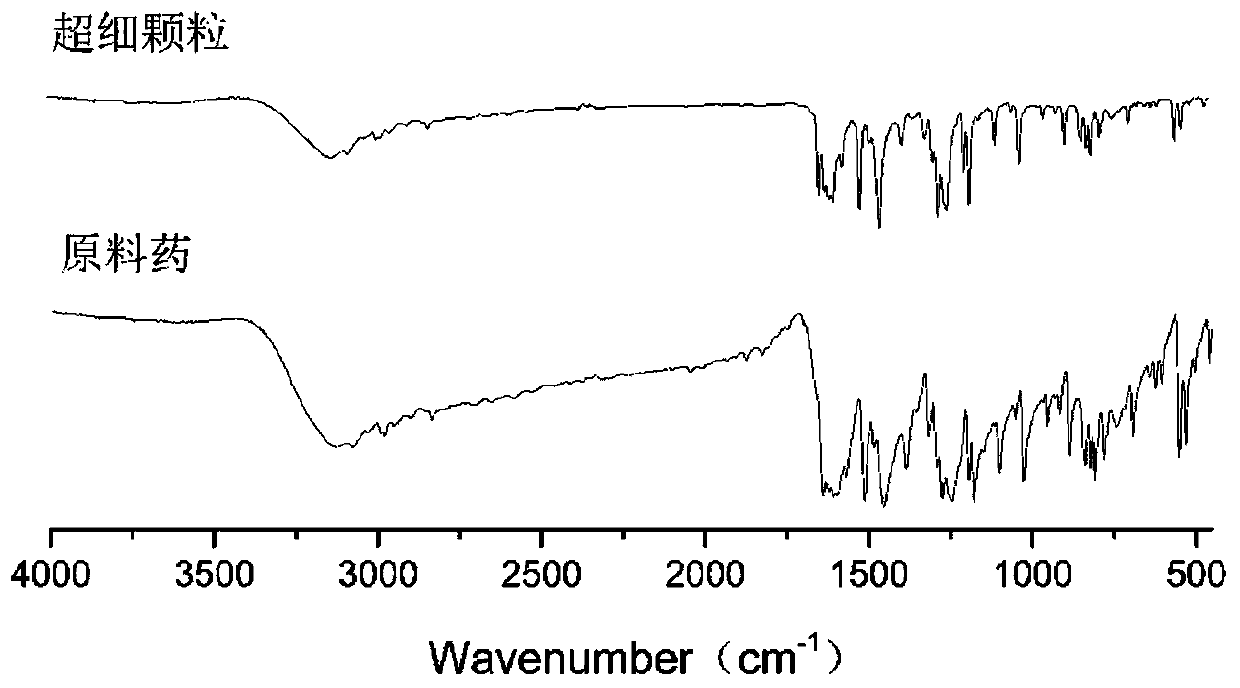
[0051] Example 2: Application of supercritical anti-solvent crystallization technology to prepare formononetin ultrafine particles under optimal process parameters
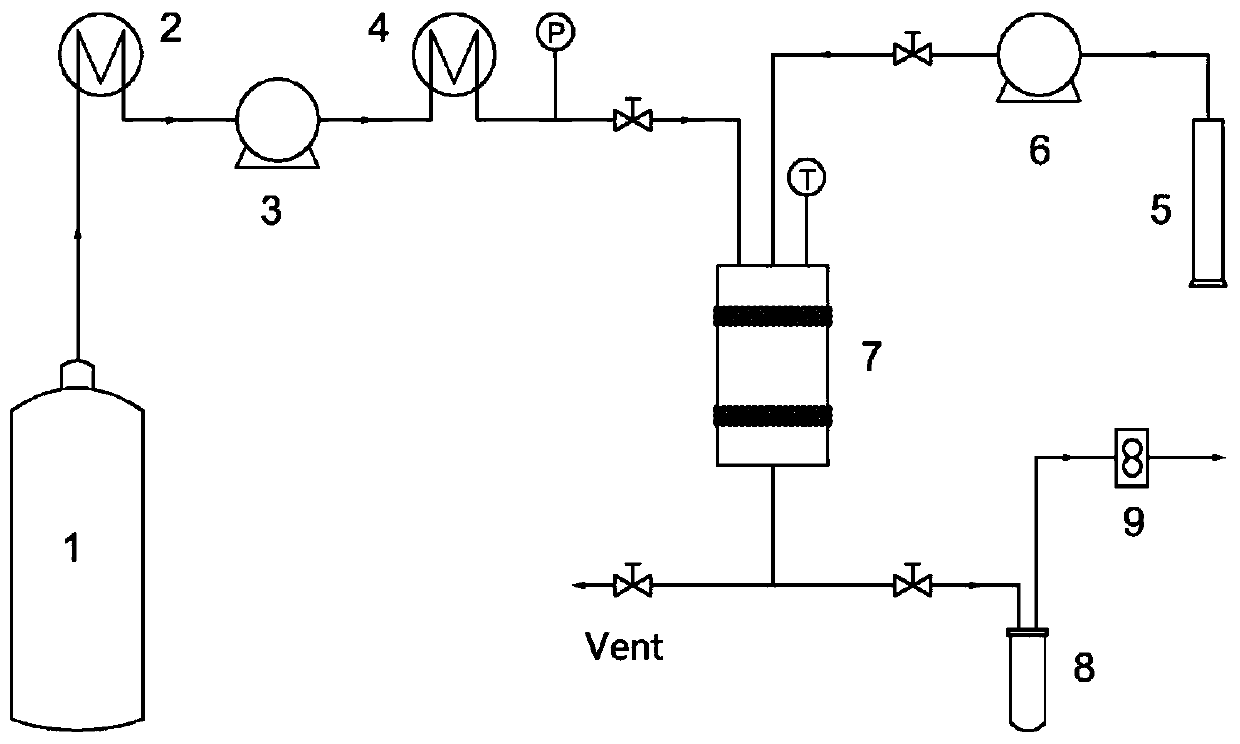
[0052] The method for preparing formononetin ultrafine particles by using supercritical anti-solvent crystallization technology comprises the following steps:
[0053] Step S1, dissolving the formononetin raw material drug in a mixed organic solvent to obtain a formononetin solution;
[0054] Step S2, adjust the temperature in the crystallization kettle to be constant, and CO 2 Pass into the crystallization kettle at a certain flow rate, and adjust the pressure of the crystallization kettle to be constant;
[0055] Step S3, continue to feed CO 2 , keep the temperature and pressure in the crystallization kettle constant, and at the same time, spray the above-mentioned formononetin solution into the kettle through the high-pressure infusion pump through the nozzle on the top of the crystallization kettle;
[0056...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com