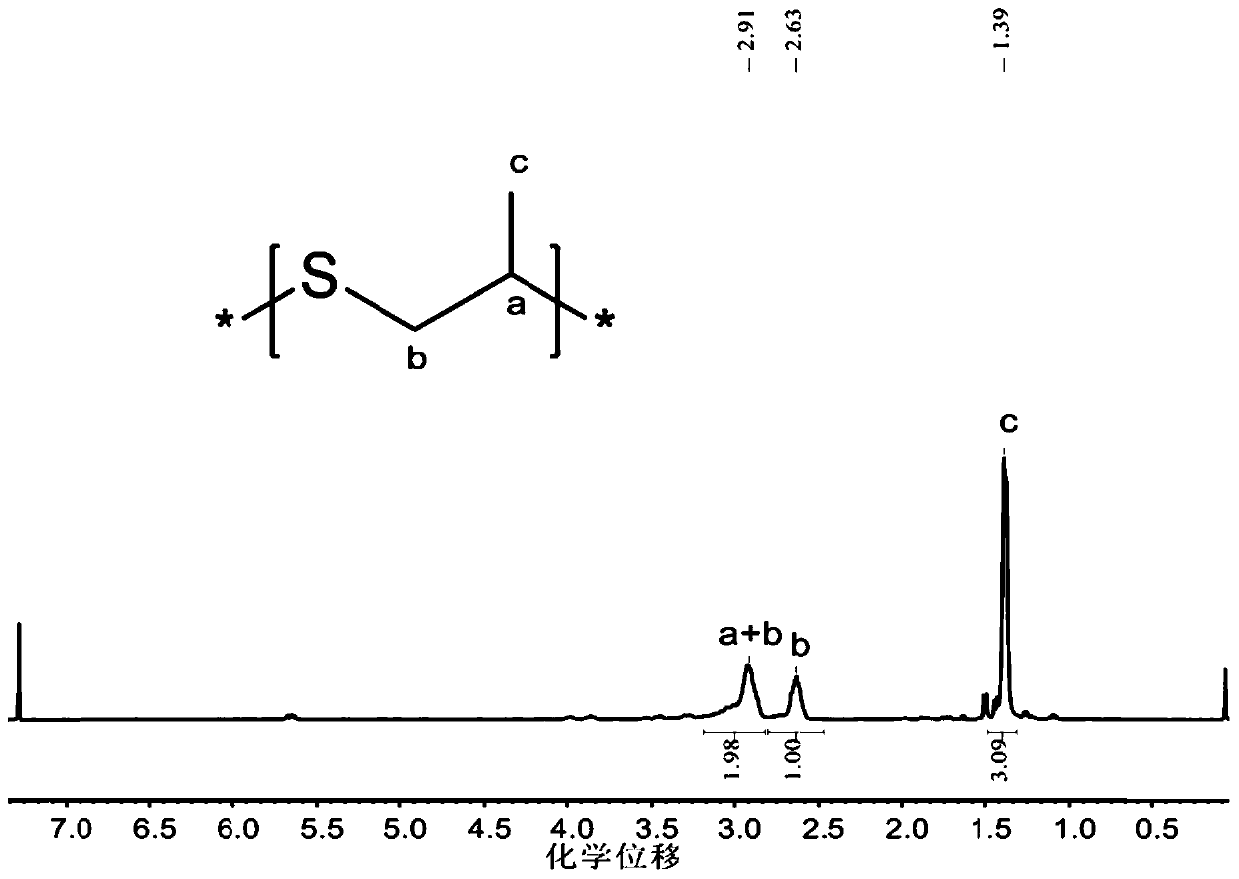
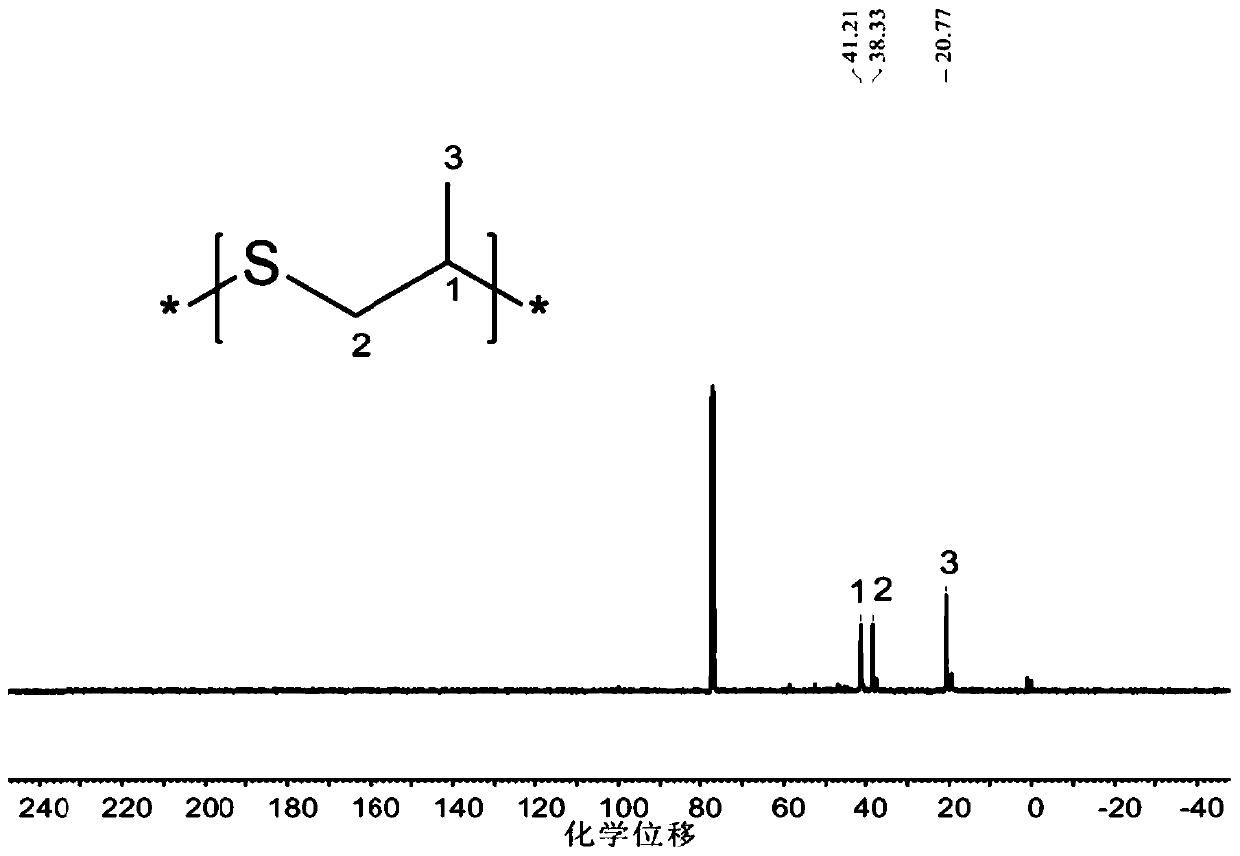
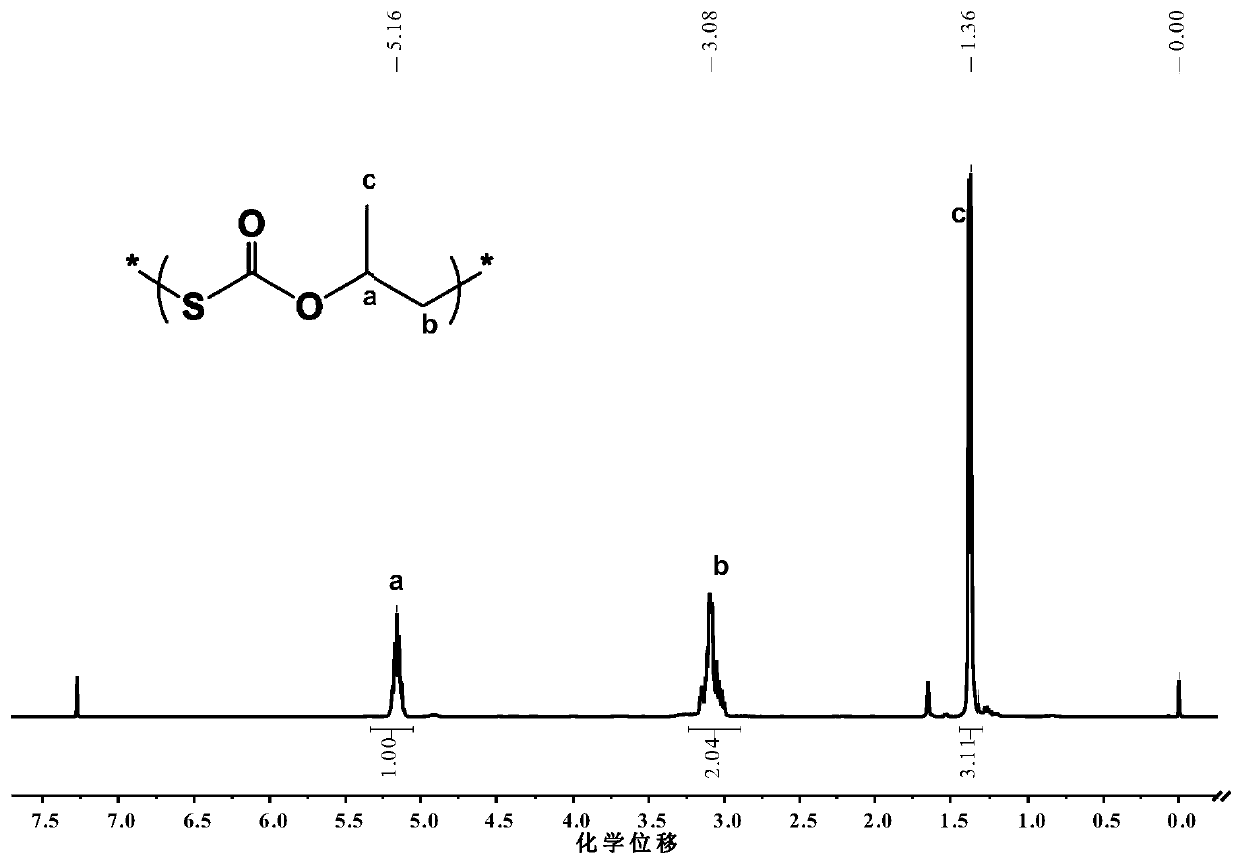
Method for preparing carbon oxysulfide and co-producing sulfur-containing polymer
A technology of carbon oxysulfide and polymers, applied in the direction of carbon oxysulfide and carbon sulfur compounds, etc., can solve the problems of waste generation, long process, increased cost, etc., achieve good universality, reduce environmental impact, and avoid purification steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 CS 2 / PO to prepare carbon oxysulfide and co-production with PO to prepare polymonothiocarbonate
[0050] Before the polymerization reaction, 10mL of reactor I and reactor II were dehydrated at 110°C for about 2 hours, cooled to room temperature in a desiccator, and connected through valves and pipelines; the valves were closed at the initial stage, and the reactors were supplied to reactor I in turn. Add some quality Lewis (Lewis) base 1 1,8-diazabicyclo[5.4.0]undec-7-decene (DBU); the molar ratio of Lewis base 1 to propylene oxide (PO) is 1 :10; add CS again 2 , PO(CS 2 with PO molar ratio 1:1) and 1 mL of toluene. Then the reactor I was closed and placed in an 80°C oil bath for 0.5h under autogenous pressure. After the reaction, the reactor I was cooled to room temperature. Several masses of PO, Lewis base 11,8-diazabicyclo[5.4.0]undec-7-decene (DBU) and Lewis acid triethylboron and 1 mL THF were added to Reactor II; Lewis base 1 Reactor II was closed...
Embodiment 2
[0052] Example 2 CS 2 / PO to prepare carbon oxysulfide and co-production with PO to prepare polymonothiocarbonate
[0053] Before the polymerization reaction, 10mL of reactor I and reactor II were dehydrated at 110°C for about 2 hours, cooled to room temperature in a desiccator, and connected through valves and pipelines; the valves were closed at the initial stage, and the reactors were supplied to reactor I in turn. Add some quality Lewis base 2 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD); the molar ratio of Lewis base 2 and propylene oxide (PO) is 1 / 500; Rejoin CS 2 , PO(CS 2 with PO molar ratio 3:1) and 1 mL of toluene. Then the reactor I was closed and placed in an oil bath at 80° C. for 6.0 h under autogenous pressure. After the reaction, the reactor I was cooled to room temperature. Several masses of PO, Lewis base 2 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) and Lewis acid triethylboron and 1 mL of DMF were added to Reactor II; Lewis base 2 The molar ratio to propyl...
Embodiment 3
[0054] Example 3 CS 2 / PO to prepare carbon oxysulfide and co-production with PO to prepare polymonothiocarbonate
[0055] Before the polymerization reaction, the 10mL two reactors I and II were dehydrated at 110°C for about 2 hours, cooled to room temperature in a desiccator, and connected through valves and pipelines; the valves were closed at the initial stage, and the reaction Add some quality Lewis base 3 sodium methylate in the device I; the molar ratio of Lewis base 3 and propylene oxide (PO) is 1 / 500; add CS 2 , PO(CS 2 with PO molar ratio 10:1) and 1 mL of toluene. Then the reactor I was closed and placed in an oil bath at 100° C. for 0.5 h under autogenous pressure. After the reaction, the reactor I was cooled to room temperature. Add several masses of PO, Lewis base 3 sodium methoxide and Lewis acid tributylboron and 1 mL THF to reactor II; the molar ratio of Lewis base 3 to propylene oxide (PO) is 1 / 400, and the molar ratio of Lewis acid to The ratio is 1 / 5 an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com