Compound for immobilizing proteins and method for immobilizing proteins
A compound and protein technology, applied in the field of immobilized proteins, can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] specific implementation plan
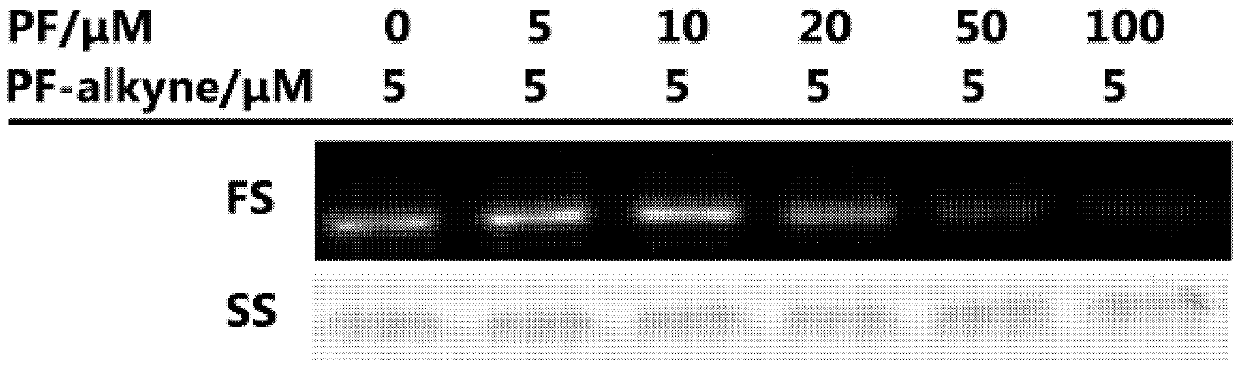
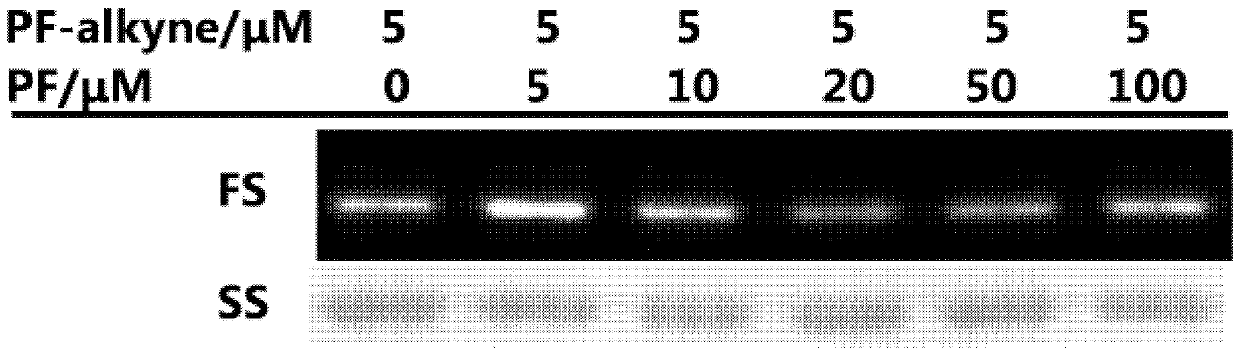
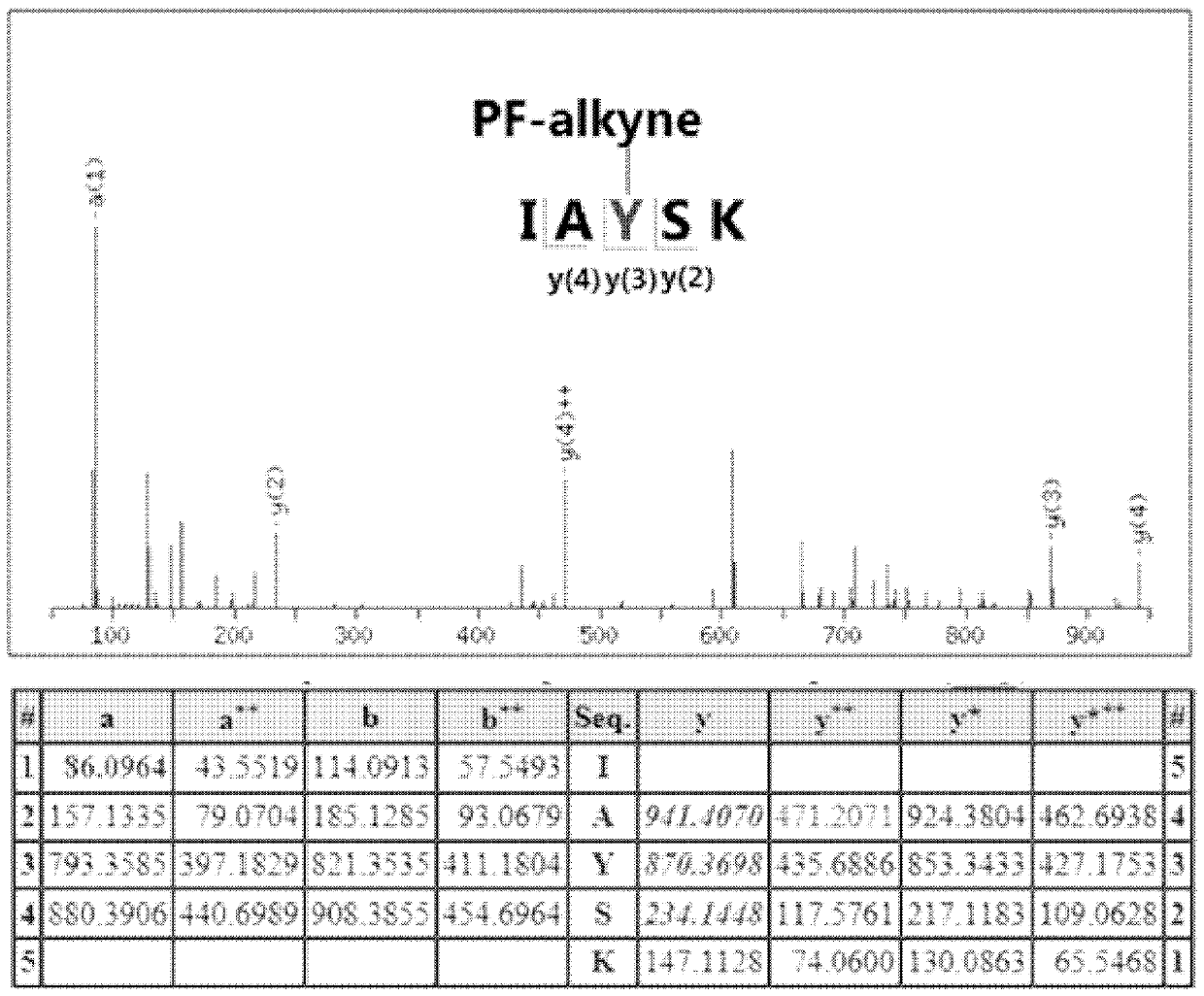
[0034] In the following examples, all the reagents used in the experiments were purchased from reagent companies and no further processing was performed. Dichloromethane was dried over calcium hydride to remove water, and tetrahydrofuran was dried over sodium block to remove water. All reactions were monitored by 0.25 mm thick TLC silica gel plates and UV light. The silica gel used in flash column chromatography is 200-300 mesh silica gel powder. The Bruker 400M and 500M NMR instruments were used for the collection of hydrogen and carbon spectra, with trimethylsilicon (δH 0.00 and δC 0.00) as a reference. The 19F spectrum adopts a Bruker 400 mega-nuclear magnetometer, and fluorotrichloromethane (δF 0.00) is used as a reference. The 32P spectrum adopts a Bruker 400 M NMR instrument, with 85% phosphoric acid (δP 0.00) as a reference. High-resolution mass spectrometry data were collected using a quadrupole time-of-flight tandem mass spect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com