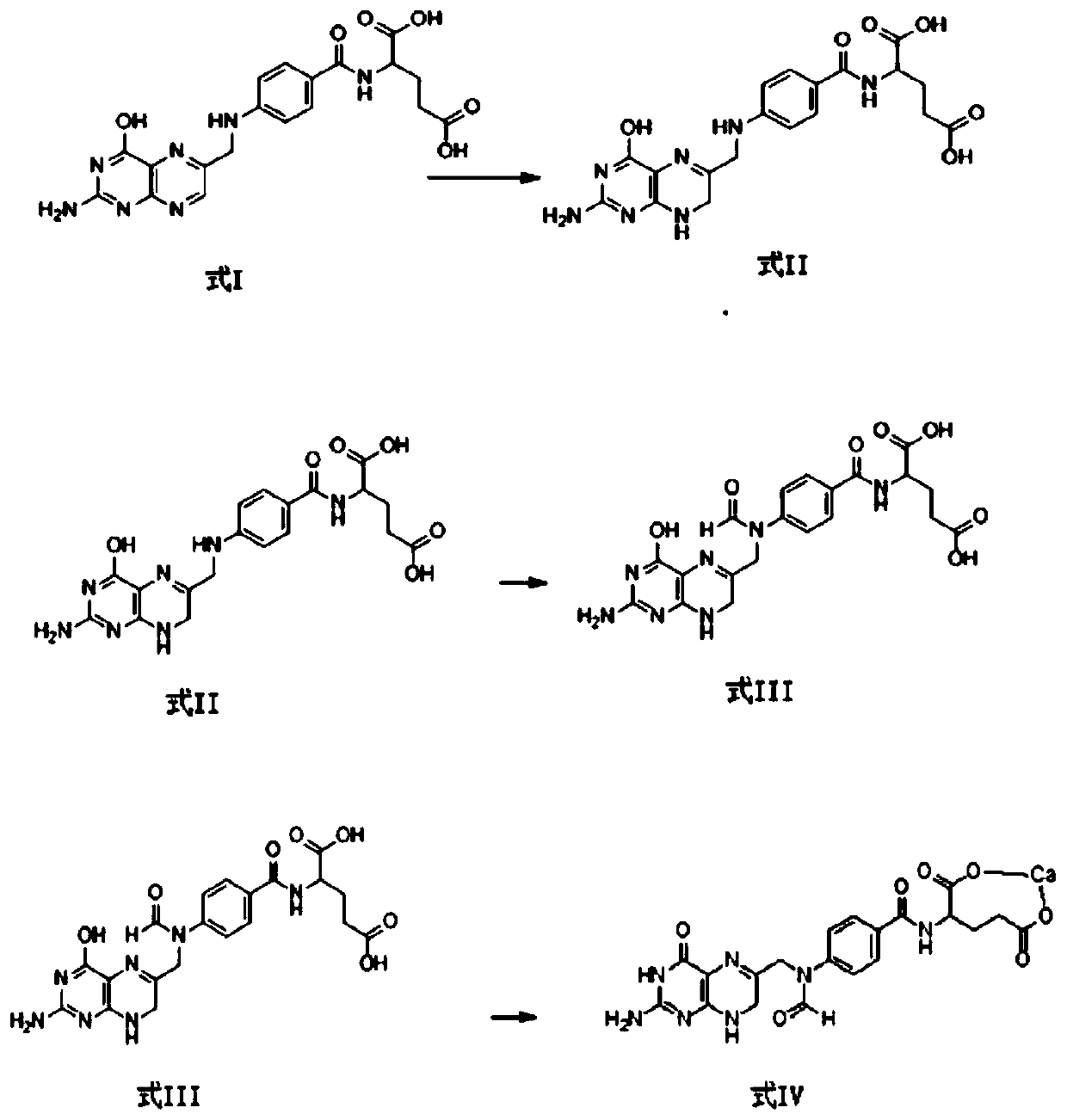
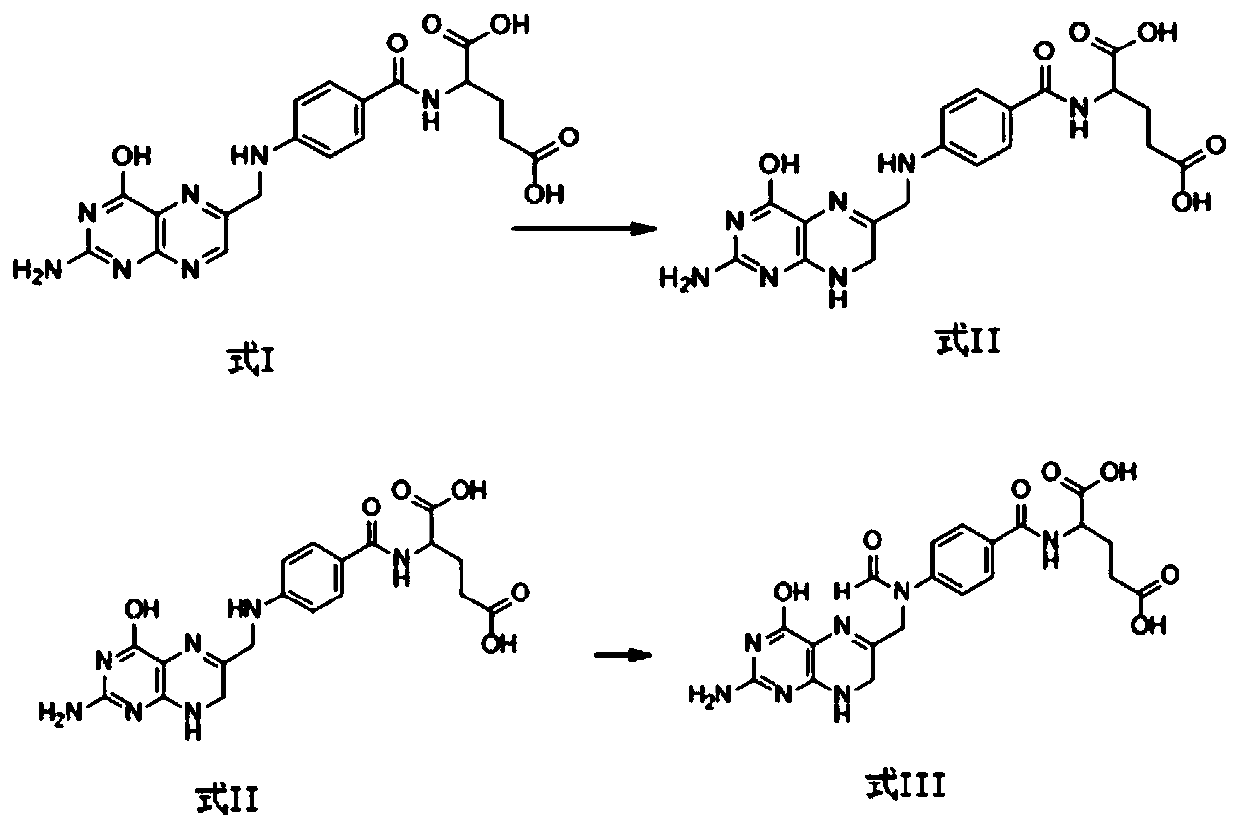
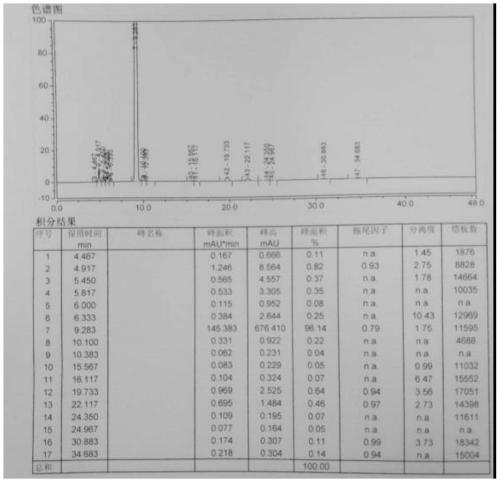
Preparation method of calcium levofolinate impurity and impurity calcium salt
A technology for calcium levofolinate and impurities, applied in the field of chemical pharmacy, can solve the problems of low purity of 10-formyl dihydrofolate, etc., and achieve the effects of low equipment requirements, simple process and high calibration content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Dissolve 10g (22.7mmol) of folic acid in 200ml of water, adjust the pH of the reaction solution to 8.5 with aqueous sodium hydroxide solution, stir to dissolve, add 17.2g (90.7mmol) of sodium metabisulfite, heat up to 30°C and keep it for 2 hours, then cool down to 0°C At ~5°C, add hydrochloric acid dropwise, adjust the pH of the reaction solution to 0.5, keep warm at 0°C-5°C for 24 hours, filter, and dry under reduced pressure at 50°C to obtain 8.6 g of dihydrofolic acid, with a yield of 79.6%.
[0034] Dissolve 8.6g (19.4mmol) of dihydrofolate in 104g of formic acid, keep it warm at 25°C and react for 30 hours. After the reaction is complete, distill the formic acid under reduced pressure at 50°C, add 86ml of purified water, stir and crystallize, filter, and dry at 50°C , to obtain 7.0 g of 10-formyl dihydrofolate, with a yield of 83.1%.
Embodiment 2
[0036] Dissolve 8.6g (19.4mmol) of dihydrofolate in 104g of formic acid, keep warm at 25°C and react for 30h. After the reaction is complete, distill the formic acid under reduced pressure at 50°C, add 86ml of purified water, stir and crystallize, and filter to obtain 10- Formyl dihydrofolate. Dissolve 10-formyl dihydrofolic acid in 86ml of purified water, adjust the pH of the solution to 7.0 with aqueous sodium hydroxide solution, add 1.1g (9.7mmol) of calcium chloride, react at room temperature for 1 hour, filter, and dry under pressure at 50°C to obtain 6.3g calcium 10-formyl dihydrofolate, yield 67.7%.
Embodiment 3
[0038] Dissolve 15g (34.0mmol) of folic acid in 300ml of water, adjust the pH of the reaction solution to 8.0 with aqueous potassium hydroxide solution, stir to dissolve, add 21.2g (204mmol) of sodium bisulfite, heat up to 40°C for 2 hours, and cool down to 0 ℃~5℃, add hydrochloric acid dropwise, adjust the pH of the reaction solution to 0.8, keep warm at 0℃~5℃ for 24 hours, filter, and dry under reduced pressure at 50℃ to obtain 10.7g of dihydrofolic acid with a yield of 71.0%.
[0039] Dissolve 10.7g (24.2mmol) of dihydrofolate in 257g of formic acid, keep warm at 40°C and react for 24h. After the reaction is complete, distill the formic acid under reduced pressure at 50°C, add 107ml of purified water, stir and crystallize, filter, and depressurize at 50°C After drying, 8.6 g of 10-formyl dihydrofolate was obtained, with a yield of 81.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com