Furocoumarin compound, and preparation method and medical application thereof
A furanocoumarin and a technology for its application, which are applied in the preparation of new compounds, the preparation of furanocoumarins, and the field of furanocoumarins, can solve the problem that the chemical drug treatment of hypertension cannot meet expectations and reduce the The problems of drug resistance, chemical drug toxicity, etc
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Stir the dichloromethane solution of chlorosulfonic acid (100 mmol / 20 mL) in an ice bath for 30 min, slowly add xanthotoxin (10 mmol) in batches, and continue the ice bath for 1 h;
[0024] 2) After the reaction is completed, the solvent is removed under reduced pressure, and then slowly added dropwise to the ice-water mixture, filtered with suction, washed, and dried to obtain the white chlorosulfonated product of xanthotoxin;
[0025] 3) Dissolve 5-aminotetrazole (1 mmol) and N,N-diisopropylethylamine (2 mmol) in 10 mL of dichloromethane under an ice bath;
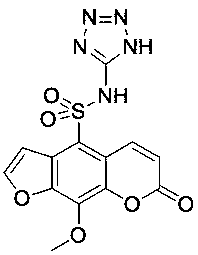
[0026] 4) After cooling down for 30 minutes, add the chlorosulfonated pepper toxin (0.8 mmol) prepared in step 3), continue the reaction for 2 hours, add water for extraction, wash with 10% hydrochloric acid, saturated sodium bicarbonate and saturated saline successively, and filter with suction , dried, and obtained compound S31 by column chromatography; named: 9-methoxy-7-oxo-N-(1 H -tetrazol-5-yl)-7 H -fur...
Embodiment 2
[0029] pharmaceutical composition
[0030] Formulation for 1000 tablets each containing 100 mg of active ingredient:
[0031] Compound S31------------------------------------------------ ---------100g
[0032] Hydroxypropyl Cellulose --------------------------------------------- -----------2g
[0033] wheat starch------------------------------------------------ ------------10g
[0034] lactose------------------------------------------------- --------------100g
[0035] Magnesium stearate ----------------------------------------------- -------------3g
[0036] talc------------------------------------------------- ------------3 g
[0037] The dosage used should be adapted to the nature and severity of the disease, the route of administration and the age and weight of the patient. The daily dose varies between 0.1 mg-1.0 g, and can be administered once or several times.
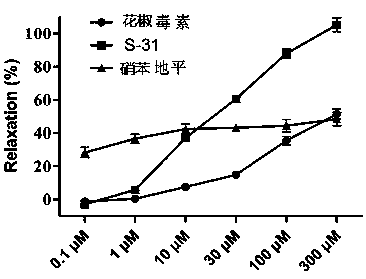
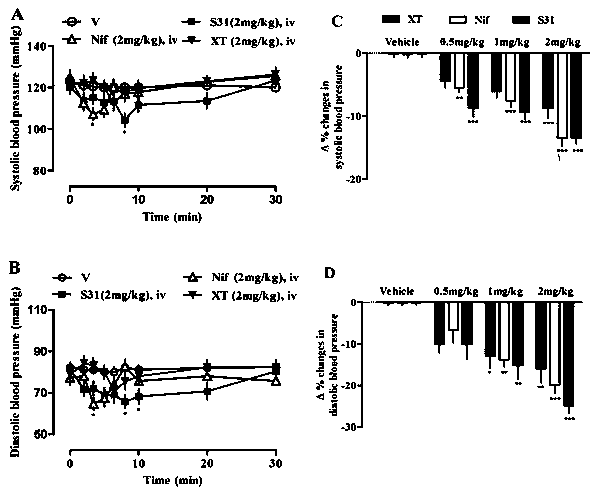
[0038] Further prove the medical purposes of compound of the present invention by following test:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com