Protein degradation targeting BCR-ABL compound and application thereof to resisting tumors
A technology of compounds and solvates, applied in the field of protein degradation of compounds targeting BCR-ABL fusion proteins, can solve unseen problems such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
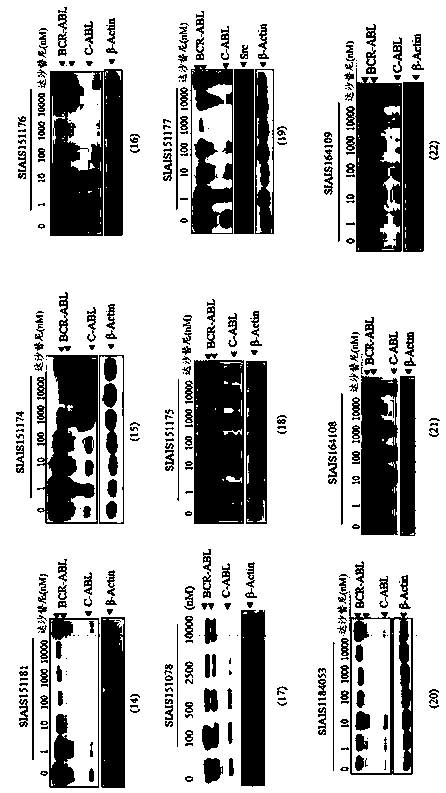
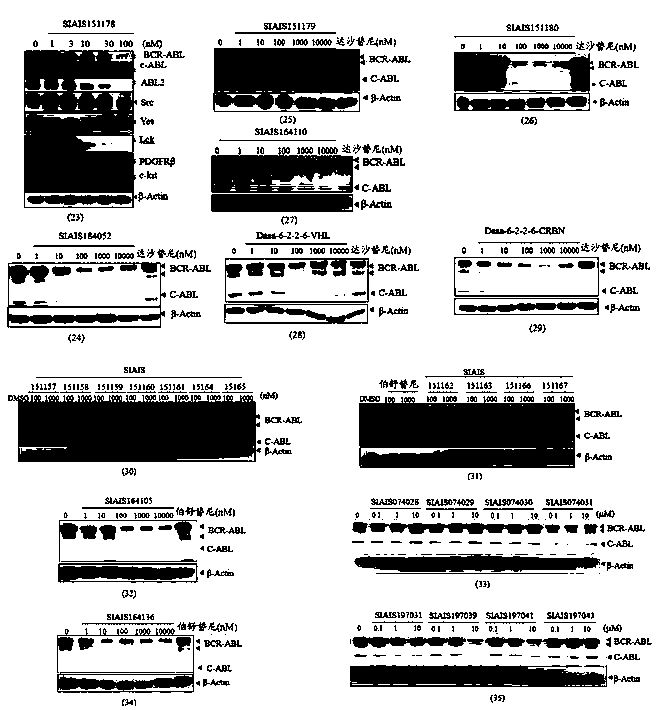
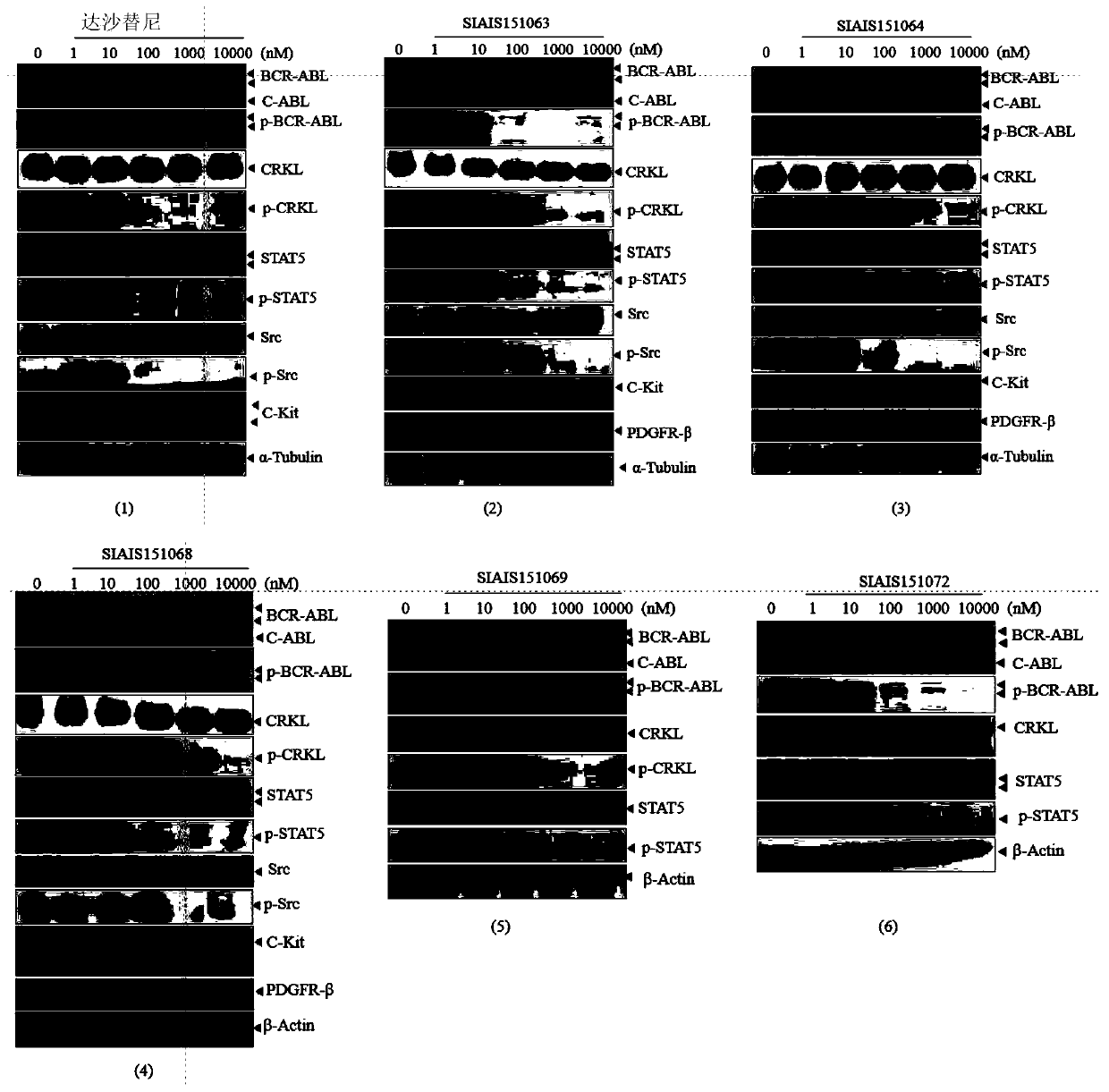
Image
Examples
Embodiment
[0162] In the following description, numerous specific details are set forth in order to provide a thorough understanding of the present disclosure. The present disclosure may be practiced without some or all of these specific details. In other instances, well known process operations have not been described in detail in order not to unnecessarily obscure the present disclosure. While the disclosure will be described in conjunction with specific embodiments, it will be understood that they are not intended to limit the disclosure to those embodiments.
[0163] The following abbreviations are used throughout the specification and examples:
[0164] Boc tert-butoxycarbonyl
[0165] n-BuOH n-Butanol
[0166] t BuOH tert-butanol
[0167] Con. Concentration
[0168] DCM dichloromethane
[0169] DME ethylene glycol dimethyl ether
[0170] DMF N,N-Dimethylformamide
[0171] DMSO dimethyl sulfoxide
[0172] DIPEA N,N-Diisopropylethylamine
[0173] EDCI carbodiimide
[0174...
preparation example 1
[0207] Intermediate Preparation Example 1: Preparation of Dasatinib Derivatives
[0208]
[0209] plan 1
[0210] N-(2-Chloro-6-methylphenyl)-2-((2-methyl-6-(piperazin-1-yl)pyrimidin-4-yl)amino)thiazole-5-yl was prepared according to Scheme 1 Formamide (SIAIS151055):
[0211] N-(2-chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide (1.0g, 2.54mmol), Add anhydrous piperazine (1.31g, 15.21mmol), N,N-diisopropylethylamine (4.9g, 38.0mmol) and anhydrous n-BuOH (8mL) into a 30mL microwave reaction tube, and stir at room temperature After 10 minutes, argon gas was slowly blown into the microwave tube, and after sealing, the reaction tube was put into a microwave reactor, and the temperature was slowly raised to 120° C., and stirred for 1 h. The reaction solution was lowered to room temperature and left overnight, a large amount of white solids precipitated, suction filtered, the filter cake was washed twice with anhydrous n-butanol, and the ...
preparation example 2
[0212] Intermediate Preparation Example 2: Preparation of Bosutinib Derivatives
[0213]
[0214] Scenario 2
[0215] Preparation of 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(piperazin-1-yl)propoxy)quinone according to Scheme 2 Phenyl-3-carbonitrile (SIAIS151151):
[0216] 7-(3-chloropropoxy)-4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-3-cyanoquinoline (1.0g, 2.14mmol), anhydrous piperazine (0.93g, 10.7mmol), sodium iodide (0.4g, 2.14mmol) and ethylene glycol dimethyl ether (8mL) were added together in a 30mL microwave reaction tube, stirred at room temperature for 10min, Then argon gas was slowly bubbled into the microwave tube, the reaction tube was placed on the microwave reactor, raised to 95° C. and stirred for 1 h. The reaction solution was lowered to room temperature, the reaction solvent was evaporated under reduced pressure, then 20 mL of saturated sodium bicarbonate solution was added, extracted with ethyl acetate (4×50 mL), the organic phases...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com