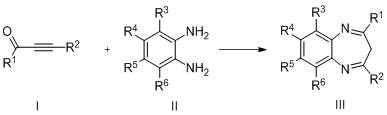
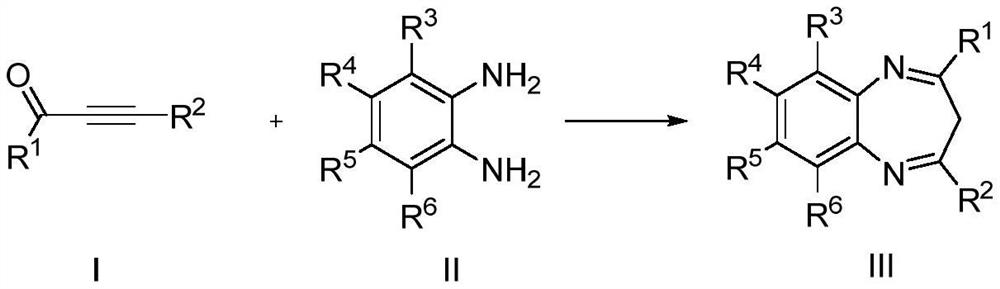
Method for preparing benzodiazepine derivatives catalyzed by dichlorotitanocene
A dichloro-titanocene catalysis, technology of dichloro-titanocene, applied in the direction of chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., to achieve a wide range of biological activities and medicinal value, non-toxic solvents, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
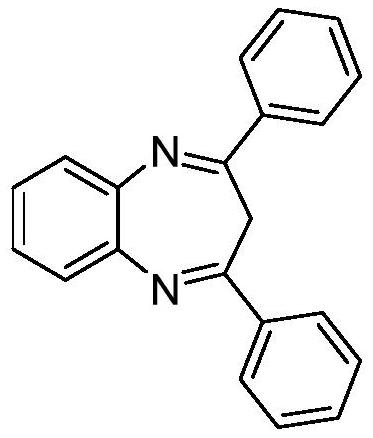
Embodiment 1
[0016] Preparation of 2,4-diphenyl-3-H-1,5-benzodiazepine with the following structural formula
[0017]
[0018] Add 0.0025g (0.01mmol) titanocene dichloride, 0.0033g (0.02mmol) isophthalic acid, 0.065g (0.6mmol) o-phenylenediamine, 0.103g (0.5mmol) 1,4-diamine to the reaction flask Phenyl-3-butyn-2-one, 1 mL of ethanol, stirred and reacted at room temperature for 5 hours, stopped the reaction, removed the ethanol by rotary evaporation, and separated with a silica gel column (the eluent was that the volume ratio of petroleum ether and ethyl acetate was 10: 1) to obtain 2,4-diphenyl-3-H-1,5-benzodiazepine with a yield of 96% and the spectral data of the product: 1 H NMR (600MHz, CDCl 3 )δ7.95-7.81(m,4H),7.53(dd,J=6.1,3.5Hz,2H),7.387.31(m,6H),7.27(dd,J=6.1,3.5Hz,2H),7.18 (s, 0H), 1.53 (s, 1H); 13 C NMR (101MHz, CDCl 3 )δ154.26,140.83,137.34,130.69,128.83,128.77,128.22,125.54,35.01.
Embodiment 2
[0024] Preparation of 2-(4-Fluorophenyl)-4-phenyl-3-H-1,5-benzodiazepine of formula
[0025]
[0026] In this example, the 1,4-diphenyl-3-butane used in Example 1 was replaced with an equimolar amount of 1-(4-fluorophenyl)-4-phenyl-3-butyn-2-one Alkyn-2-one, other steps were the same as in Example 1, to obtain 2-(4-fluorophenyl)-4-phenyl-3-H-1,5-benzodiazepine in 98% yield, The spectral data of the product are: 1 H NMR (400MHz, CDCl 3 )δ7.97(ddd,J=7.7,4.9,2.0Hz,4H),7.62(dtd,J=7.5,3.5,1.8Hz,2H),7.43(dd,J=5.2,2.0Hz,3H),7.35 (dd, J=6.2, 3.5Hz, 2H), 7.09 (t, J=8.6Hz, 2H); 13 C NMR (101MHz, CDCl 3 )δ165.55,163.05,153.95,152.78,140.72,140.59,137.22,133.58,133.55,130.74,130.37,130.28,128.79,128.72,128.18,128.14,125.56,134.78
Embodiment 3
[0028] Preparation of 2-(4-chlorophenyl)-4-phenyl-3-H-1,5-benzodiazepine of formula
[0029]
[0030] In this example, the 1,4-diphenyl-3-butane used in Example 1 was replaced with an equimolar amount of 1-(4-chlorophenyl)-4-phenyl-3-butyn-2-one Alkyn-2-one, other steps were the same as in Example 1, to obtain 2-(4-chlorophenyl)-4-phenyl-3-H-1,5-benzodiazepine in 98% yield, The spectral data of the product are: 1 H NMR (400MHz, CDCl 3 )δ7.96(dd,J=7.5,2.3Hz,2H),7.91(d,J=8.6Hz,2H),7.65-7.57(m,2H),7.46-7.41(m,3H),7.39(s ,1H),7.38-7.32(m,3H); 13 C NMR (101MHz, CDCl 3 )δ153.90,152.68,140.76,140.47,137.15,136.91,135.70,130.78,129.45,128.95,128.80,128.73,128.16,125.69,125.58,34.82.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com